X射线衍射(XRD)是广泛用于分析锂离子电池正极材料组成和原子尺度结构的表征技术[9]。然而原始XRD谱图得到的仅是简单粗略的结构信息, 通过XRD结构精修可以从衍射数据中得到更详细和准确的结构信息。Rietveld精修是应用较为广泛的XRD结构精修方法, 是由荷兰Petten反应堆中心的研究员Hugo M Rietveld[10]在1967年用中子衍射数据精修结构参数时提出的一种全谱线性拟合数据处理方法。在锂离子电池正极材料的结构特征研究中, 通过XRD Rietveld精修可以明确正极材料的详细结构信息, 包括晶体结构类型、晶胞参数、关键原子的占位信息等, 结合化学/电化学性能研究, 明晰结构特征对性能的作用规律, 进而建立结构与性能之间的“构-效”关系, 为正极材料的改性研究提供指导, 对推动锂离子电池正极材料的发展具有深远意义。
本文系统综述了XRD Rietveld精修的核心要点及其近年来在锂离子电池正极材料中的应用, 主要围绕聚阴离子型、层状结构、尖晶石型、富锂锰基等典型正极材料论述了XRD Rietveld精修在正极材料合成、退化与衰减、结构改性中的应用, 并总结了其在建立正极材料结构与性能间“构-效”关系方面发挥的作用。最后, 讨论了XRD Rietveld精修在正极材料结构解析方面的不足及未来发展方向, 包括与其他局域结构表征手段的高度有机结合, 发展更高强度的X射线自由电子激光等, 以及它们对深入研究高能锂电正极材料的贡献。
1 X射线衍射技术
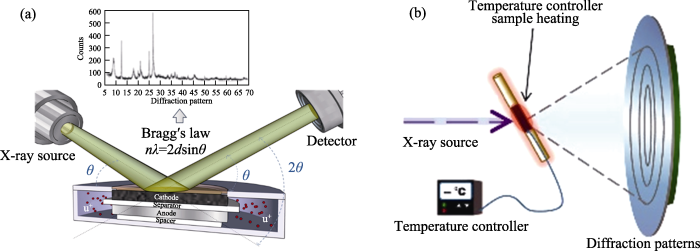
常规的实验室X射线衍射仪中, 来自灯丝中的电子束轰击到靶上, 产生X射线。X射线波长与靶材的原子序数有关, 原子序数越大, X射线波长越短。锂离子电池正极材料的实验室级XRD测试中常见的靶材为Cu, 其他有Mo、Fe、Ni、Co、Cr、Ag和W等。
利用常规真空光电管所产生的X射线逐渐不能满足精细结构表征的需求, 因而高强度的同步辐射X射线开始进入大家的视野[13]。同步辐射光源具有高亮度、高准直性、高稳定性、宽波段覆盖范围、高偏振性和脉冲时间结构等诸多优异性能。与常规的旋转阳极X光源的Kα线相比, 同步辐射X射线光谱的亮度要高出10个数量级, 使用与之相关的高性能探测器可以快速精确地获取高信噪比、高角分辨、高能量分辨的XRD谱图, 每点的曝光时间为毫秒或更短[14], 因此其在时间分辨研究和原位环境中具有特殊优势。结合Rietveld结构精修方法, 同步辐射X射线衍射技术可以更精确地解析锂离子电池正极材料的晶体结构信息, 目前已经被广泛采用。
图1
图2
2 XRD Rietveld结构精修
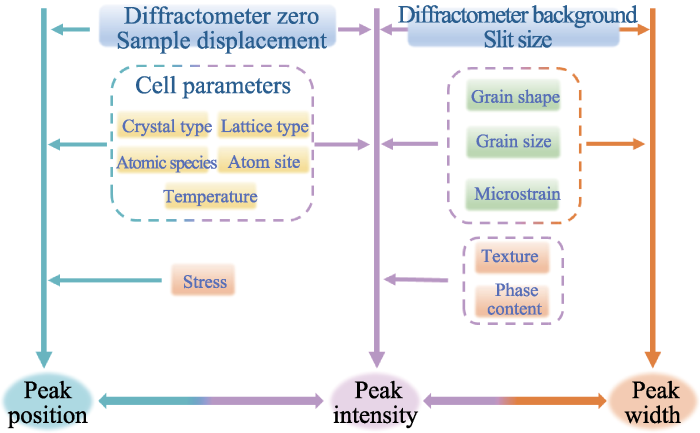
晶胞参数是影响衍射峰位和峰强的重要因素, 已知物质的晶体结构(晶体类型、空间群、原子占比和原子坐标等), 就可以计算该物质的X射线衍射谱的峰位和强度。在此基础上进一步赋予样品位移、背景线形状、温度影响(温度因子)以及择优取向(织构)等因素, 计算得到的衍射图谱以一定的峰型函数(峰宽、峰形和歪斜因子)进行叠加, 得到该物相的理论谱。然而实际测得的衍射数据往往受到仪器误差、样品状态等诸多因素影响, 造成实测图谱与理论图谱存在差距, 因此需要进行结构精修, 使计算谱无限逼近实测图谱, 最终获得样品的实际结构信息。
式中, Wi为统计权重因子; Yoi为点i处的实测强度; Yci是点i处的计算强度。
在精修过程中, 常用Rp和Rwp评判Rietveld精修结果的可靠性, Rwp的分子即是最小二乘法拟合中得到的极小量, 可以反映拟合的优劣。一般情况下, 当Rwp<10%时, 可以认为精修结果可靠。
然而, 只依据剩余方差因子(Rp与Rwp)评判精修结果并不严谨, 因为有时会出现“伪收敛”现象。在精修过程中还需要关注精修结构模型的化学合理性, 如原子占比、原子坐标等。温度因子也是判断精修结果是否可靠的重要标准, 一般精修得到的温度因子随原子序数增加而减小。
此外, 还可以通过拟合优度来评价全谱拟合, 定义为[22]:
式中, Rexp为Rwp的期望值,
Rietveld精修函数包括峰型函数、峰宽函数及本底函数, 其中选择合理的峰型函数对Rietveld精修过程至关重要[23]。在锂离子电池正极材料的精修中应用较广泛的峰型函数为Pseudo-Voigt(P-V)函数, 即高斯峰型和洛伦兹峰型的线性组合。
式中,
其中, U、V、W为峰宽参数。各衍射峰的半高宽Hk因θhkl而异, 一般较大的θhkl对应的Hk也大。
在锂离子电池正极材料的精修过程中, 建立结构模型初期可以根据电感耦合等离子测试(ICP)结果简单计算各个原子的含量, 特别是掺杂原子含量, 赋予初始结构模型中掺杂原子的占比。
3 XRD Rietveld精修在锂离子电池正极材料中的应用
3.1 正极材料的结构与性能
表1 常见的锂离子电池正极材料的结构及性能[24⇓⇓⇓-28]
Table 1
| Cathode material | Crystal structure | Space group | Cell parameter | Atom site | Theoretical specific capacity/(mAh·g-1) | Working voltage/ V (vs. Li+/Li) | |
|---|---|---|---|---|---|---|---|
| LiFePO4 | Olivine | Pnma | a≠b≠c | Li | 4a | 170 | 3.4 |
| Fe | 4c | ||||||
| O | 4c/8d | ||||||
| LiMn2O4 | Spinel | Fd-3m | a=b=c | Li | 8a | 148 | 4 |
| Mn | 16d | ||||||
| O | 32e | ||||||
| LiCoO2 | Layer | R-3m | a=b≠c | Li | 3a | 274 | 3.9 |
| Co | 3b | ||||||
| O | 6c | ||||||
| LiNixCoy(Mn/Al)1-x-yO2 | Layer | R-3m | a=b≠c | Li | 3a | 273-285 | 3.8 |
| Ni/Co/Mn/Al | 3b | ||||||
| O | 6c | ||||||
| xLi2MnO3·(1-x)LiMO2 (0<x<1, M=Ni, Co, Mn) | Layer | R-3m+ C2/m | a=b≠c | Li | 2b/2c/4h | 273-350 | 3.8 |
| Mn | 4g | ||||||
| O | 4i/4j | ||||||
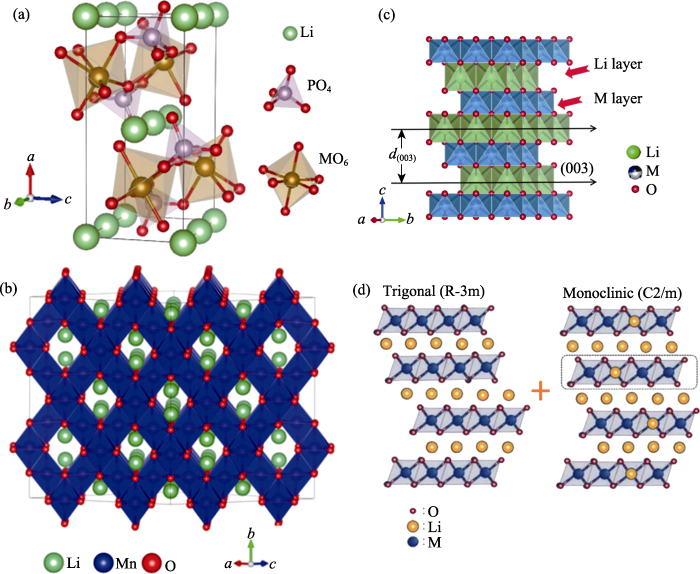
1997年, Goodenough课题组[29]最早提出并研究了聚阴离子型正极材料LiMPO4(M=Fe, Mn), 其中LiFePO4目前已经实现了商业化。LiMPO4的结构特征如图3(a)所示, PO43-聚阴离子团把Li和M原子在ac面上形成的Z字形链连接在一起, 构成LiMPO4的骨架结构, 锂离子脱嵌不会破坏其结构。一般, P-O键长决定了材料的结构稳定性, 键长越短键能越强, 结构越稳定, 有助于改善材料的循环性能; 另外, Li-O键强弱决定了Li的脱嵌难易程度, Li-O越弱, 锂离子越容易脱嵌, 有助于改善材料的倍率性能。因此, 明确晶体结构中P-O键及Li-O键是其性能研究的关键。
图3
Fig. 3
Schematic diagram of the crystal structures of cathode materials[25⇓⇓-28]
(a) LiMPO4 (M=Fe, Mn)(© 2021, IOP Publishing)[25]; (b) LiMn2O4 (© 2022, MDPI)[26]; (c) LiMO2 (M=Ni, Co, Mn, Al) (© 2021, Elsevier Ltd.)[27]; (d) xLi2MnO3·(1-x)LiMO2 (M=Ni, Co, Mn) (© 2022, Springer Nature)[28] Colorful figures are available on website
层状正极材料是目前研究最广泛的锂离子电池正极材料之一[32], 其结构特征是六方层状结构, 空间群为R-3m, 晶体结构如图3(c)所示, 锂离子和过渡金属离子分别占据3a和3b位置, 氧离子占据6c位置。沿着z轴方向, Li-O八面体和M-O八面体交替排列, 形成了锂离子传输的二维通道。(003)晶面间距代表锂离子扩散通道, 换言之, (003)晶面间距越大, 锂离子扩散越容易[33-34]。同时晶胞参数c也代表了锂层层间距的变化。而c/a反映了层状结构的有序度, 当c/a大于4.9时, 表明层状结构已形成, 数值越大表明层状结构越完整[35⇓-37]。富锂锰基正极材料是层状正极材料中的一种特殊结构, 其结构如图3(d)所示, 空间群为单斜(C2/m)及六方层状(R-3m), 结构特征与层状正极材料相似[38]。此外, 在高镍层状正极材料LiNixCoy(Mn/Al)1-x-yO2 (x>0.5)中, 由于Ni2+半径(0.069 nm)与Li+半径(0.076 nm)接近, 这极易导致Ni2+(3b)占据Li位(3a)。 XRD Rietveld精修可以计算Ni2+占据3a位置的含量, 即“Li/Ni反位”占比, “Li/Ni反位”现象会导致锂离子扩散困难, 影响材料的倍率性能[39-40]。在层状正极材料的研究中, 明确锂层层间距及“Li/Ni反位”占比是研究层状正极材料的关键环节。
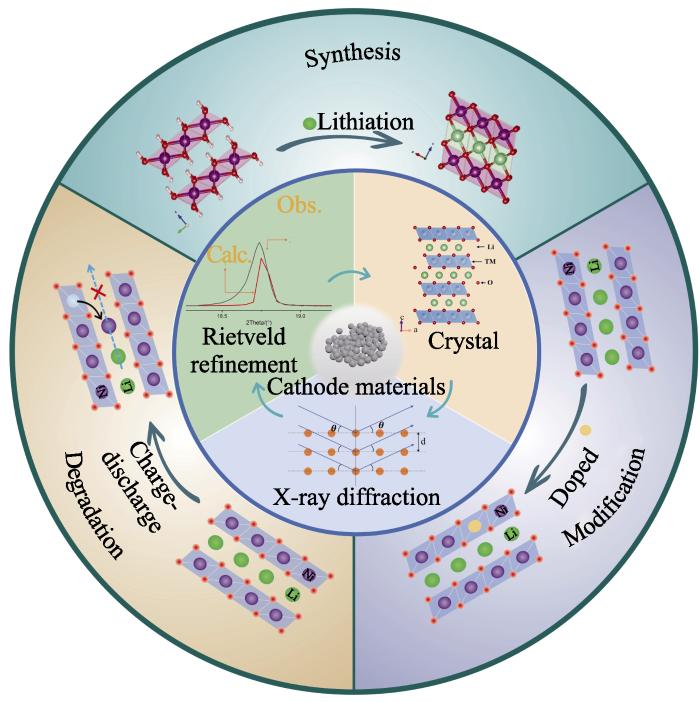
综上所述, 正极材料的晶格结构与电池性能指标密切相关, 如功率和能量密度、循环性能和安全性。因此, 深入理解结构-性能关系对于优化当前正极材料体系以及开发具有优越电化学性能的新体系正极材料至关重要。这需要研究正极材料的结构特征及其在经历化学/电化学反应时的结构演变, 包括晶格参数变化、相变和原子占位。XRD Rietveld精修是研究正极材料结构信息的必要手段, 下面重点介绍其在锂离子电池正极材料的合成、退化与衰减、结构改性研究中的应用进展, 如图4所示。
图4
图4
XRD Rietveld精修在锂离子电池正极材料中的应用
Fig. 4
Application of XRD Rietveld refinement in cathode materials for lithium-ion batteries
3.2 在正极材料合成中的应用
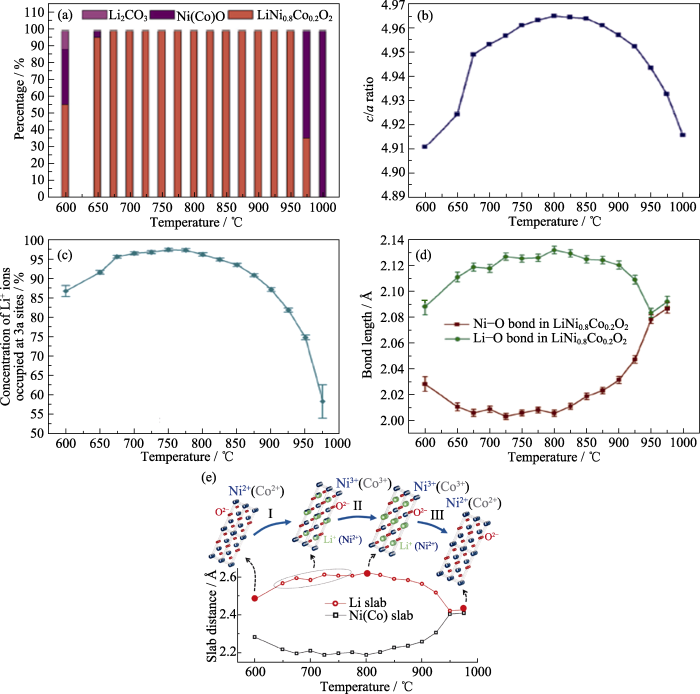
高镍层状正极材料的合成路径往往是复杂的, 特别是合成条件对高镍层状正极材料结构中阳离子有序度有明显影响, 极易导致“Li/Ni反位”。Zhao等[41]通过原位高温XRD及Rietveld精修研究了Co掺杂对层状正极材料LiNiO2合成过程中阳离子有序度的影响, 获得了与阳离子有序度相关的结构信息, 如相含量、Li占有率、局域键合。如图5(a), LiNi0.8Co0.2O2在低温及高温时均存在NiO岩盐相, 值得注意的是, 在600 ℃时已出现层状相, 比合成LiNiO2过程中的温度更低。图5(b)给出了c/a随烧结温度的变化规律, 可以看出层状结构的有序度随着温度升高表现出先升高后降低的趋势, Li占据3a位的含量表现出同样的规律(图5(c)), 随着3a位上Li含量提高, Li-O键增长, Li层间距变大, 同时, Ni-O键缩短, Ni层间距减小, 这主要是由于在过渡金属层中Ni2+含量降低。随着合成温度升高, 层状结构的有序度表现出降低的趋势, 如图5(c, d)所示, 这表明在高温(>850 ℃)和低温(<750 ℃)下, Li层间距短, 且存在较多的“Li/Ni反位”现象。综上, 在LiNi0.8Co0.2O2合成过程中, 如图5(e), 随着温度升高, 结构从岩盐相转变为层状结构, 更多的Ni2+被氧化成Ni3+, 层状结构更加有序。在800 ℃时, LiNi0.8Co0.2O2可以保持最优的层状结构特征, 然而进一步热处理则导致锂流失, Ni2+迁移到锂层, 结构转变为无序岩盐相。随着对正极材料成本的要求不断提高, 有研究者采用其他金属元素代替钴, 并研究其对合成温度的影响。例如, Weber等[42]合成了高镍无钴层状正极材料LiNiO2, LiNi0.975Mg0.025O2和LiNi0.95Al0.05O2, 借助XRD精修对比了Mg掺杂和Al掺杂对合成层状正极材料LiNiO2的影响。研究表明Mg掺杂有利于在较低的温度下获得完全锂化的正极材料, 可以降低锂化温度。由以上两个例子可见, 原位高温XRD及Rietveld精修能够揭示元素掺杂影响高镍层状正极材料的层状结构有序度及合成温度的机理。
图5
图5
LiNi0.8Co0.2O2合成过程中的结构演化[41]
Fig. 5
Structural evolution during high temperature synthesis of LiNi0.8Co0.2O2 (© 2017, Wiley-VCH)[41]
(a) Concentration of the phases Ni(Co)O, Li2CO3, and LiNi0.8Co0.2O2 at different temperatures; (b) Ratio unit cell parameters (c/a), (c) content of Li+ occupying the 3a sites (Li sites), and (d) Ni-O and Li-O bond lengths as a function of temperature; (e) Schematic diagram of the structural evolution and variation of the interlayer distances of the Li and Ni(Co) slabs during the synthesis of LiNi0.8Co0.2O2. 1 Å=0.1 nm. Colorful figures are available on website
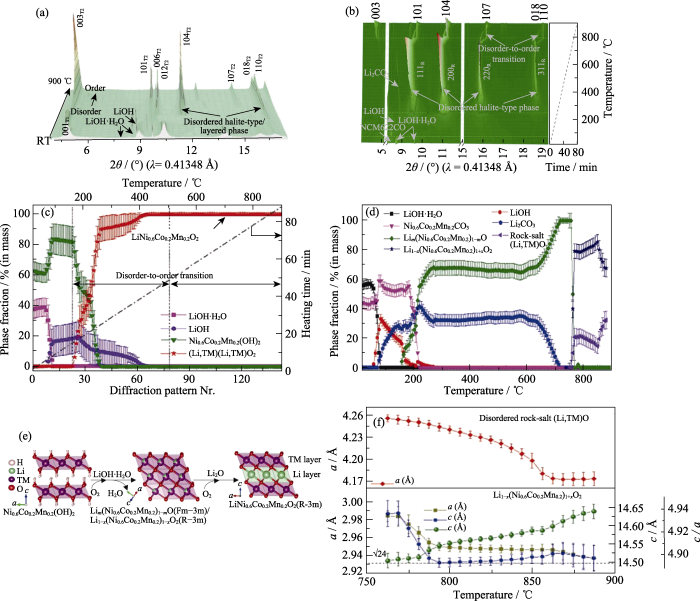
同步辐射X射线可以提供足够高的时间分辨率和满足要求的穿透深度。进一步利用此优势, 原位高温同步辐射XRD可以捕获正极材料在合成过程中的中间体, 进而有助于清晰地理解材料的合成机理。Wang等[43]通过原位高温同步辐射XRD谱图研究了高镍层状正极材料LiNi0.6Co0.2Mn0.2O2(NCM622)在高温合成过程中的结构演变机理、长程结构特征及晶体生长动力学特性。他们首先通过原位高温同步辐射XRD跟踪了前驱体Ni0.6Co0.2Mn0.2(OH)2或Ni0.6Co0.2Mn0.2CO3与锂盐混合物在不同温度下的物相变化。图6(a, b)为Ni0.6Co0.2Mn0.2(OH)2与Ni0.6Co0.2Mn0.2CO3前驱体和氢氧化锂一水合物的混合物在空气中煅烧(25~900 ℃)期间的温度分辨同步辐射XRD图谱, 结合Rietveld精修结果(图6(c, d))发现, 在以Ni0.6Co0.2Mn0.2(OH)2与Ni0.6Co0.2Mn0.2CO3两种前驱体与锂盐煅烧合成完整层状结构NCM622的过程中均出现了无序岩盐相Lim(Ni0.6Co0.2Mn0.2)1−mO (空间群为Fm-3m)和层状非化学计量比Li1−x(Ni0.6Co0.2Mn0.2)1+xO2中间体, 其结构演化过程如图6(e)所示。为了更深入地理解完全无序的岩盐相Lim(Ni0.6Co0.2Mn0.2)1−mO (Fm-3m)到部分无序的层状相Li1−x(Ni0.6Co0.2Mn0.2)1+xO2 (R-3m)的相变过程, 通过XRD精修得到了无序岩盐相与部分无序相随温度变化的晶胞参数, 如图6(f)所示, Li1−x(Ni0.6Co0.2Mn0.2)1+xO2的晶胞参数a和c在无序开始向有序转变(770~800 ℃)时都迅速降低, 说明层状结构中严重的长程阳离子无序(Li/TM)会导致平均晶胞体积和晶格参数(a和c)增加。此外, 与氢氧化物Ni0.6Co0.2Mn0.2(OH)2相比, 碳酸盐Ni0.6Co0.2Mn0.2CO3和锂盐需要更高的加热温度使Li2CO3分解。
图6
图6
正极材料LiNi0.6Co0.2Mn0.2O2的合成过程温度分辨XRD表征结果[43]
Fig. 6
Temperature-resolved XRD characterization of synthesis process of cathode material LiNi0.6Co0.2Mn0.2O2 (© 2021, Wiley-VCH)[43]
(a) In situ XRD patterns of the mixture of Ni0.6Co0.2Mn0.2(OH)2 and LiOH·H2O during heating and (c) corresponding weight fractions of different phases as a function of temperature; (b) 3D profiles of in situ XRD patterns, corresponding (d) evolution of phase fraction and (f) lattice parameters of the samples as a function of heating temperature starting from mixture of the Ni0.6Co0.2Mn0.2CO3 and LiOH·H2O; (e) Schematic illustration of structural evolution during synthesis of LiNi0.6Co0.2Mn0.2O2; In (a, b), the subscripts T1, T2 and R represent Ni0.6Co0.2Mn0.2(OH)2 (P-3m1, T1 phase), LiNi0.6Co0.2Mn0.2O2 (R-3m, T2 phase) and the rock-salt-type, respectively. 1 Å=0.1 nm Colorful figures are available on website
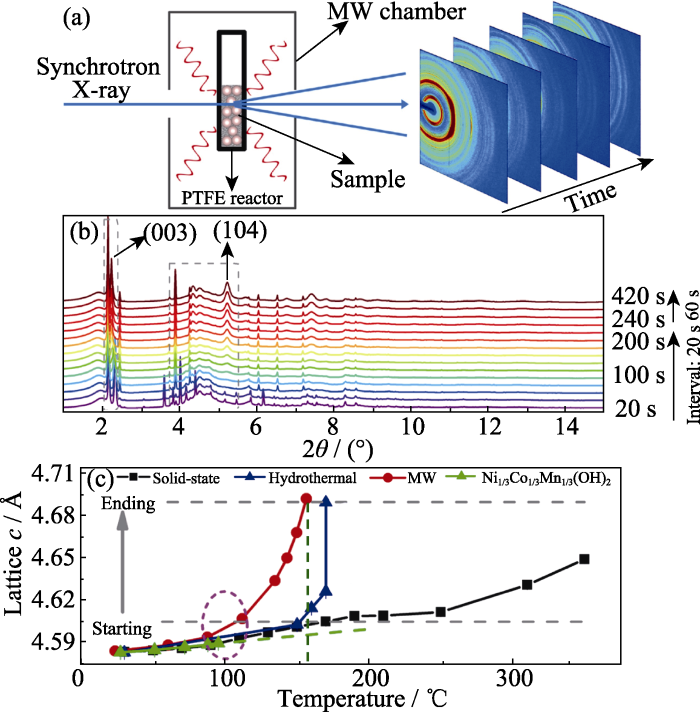
原位同步辐射XRD可以研究超快固相反应过程的晶体结构演变规律, 如应用于锂离子电池正极材料的微波合成, 精准获取合成过程中的结构演变规律及晶体学参数对于设计合成有序结构的正极材料十分重要。Zhang等[44]利用原位同步辐射XRD跟踪层状正极材料LiNi1/3Co1/3Mn1/3O2 (NCM111)的微波水热合成过程中的相变规律, 设计的微波水热合成过程原位XRD装置如图7(a)所示。他们将原料置于聚四氟乙烯(PTFE)管中, 通过改装的家用微波炉进行原位加热同步辐射XRD测试, 采用预先测定的PTFE热膨胀系数作为内部标准确定反应温度分布。微波炉满功率运行15 min, 同时记录XRD数据, 每次扫描30 s。图7(b)为不同温度下的XRD图谱, 结果表明微波水热合成中氢氧化物前驱体在160 ℃, 240 s的条件下就转变为层状氧化物产物。如图7(c)所示, 采用温度分辨XRD及Rietveld精修对比了固相合成、水热合成及微波水热合成正极材料LiNi1/3Co1/3Mn1/3O2和前驱体Ni1/3Co1/3Mn1/3(OH)2的晶胞参数c随温度的变化规律。水热合成和微波水热合成时, c从0.461 nm变化至0.469 nm, 但是微波水热合成比水热合成所需的温度明显更低。值得注意的是, 固相合成的晶胞参数c在350 ℃以内仍然较低, 即固相反应合成正极材料需要更高的温度, 表明微波水热合成的反应速率远高于固相合成和水热合成。这项研究通过原位同步辐射XRD及结构精修解析了微波超快合成的晶体结构变化规律, 进一步揭示了微波水热合成过程中的靶向能量传输机制。
图7
图7
LiNi1/3Mn1/3Co1/3O2(NCM111)的微波水热合成原位XRD表征结果[44]
Fig. 7
In-situ XRD characterization of microwave (MW) hydrothermal synthesis of NCM111(© 2020, AAAS) [44]
(a) Schematic illustration of the experimental setup specialized for fast synchrotron X-ray probing of the microwave hydrothermal synthesis; (b) Time resolved synchrotron XRD patterns during MW hydrothermal synthesis of NCM111; (c) Lattice parameter c of the Ni1/3Co1/3Mn1/3(OH)2 precursor (green) as a function of temperature during solid-state synthesis (black), hydrothermal synthesis (blue), and MW hydrothermal synthesis (red). 1 Å=0.1 nm. Colorful figures are available on website
3.3 在正极材料退化与衰减研究中的应用
正极材料在理想的电化学过程中, 随着锂离子的可逆嵌入和脱出, 其晶格结构会周期性地收缩和扩张。然而, 在实际应用中, 晶格结构的可逆性会受到诸多因素影响, 包括材料的内在性质、脱锂量、充放电电流密度等。原位电化学XRD谱图可以实时跟踪正极材料在充放电过程中晶格参数的变化规律, 有助于理解其退化和衰减机理, 本节讨论一些近期的相关实例。
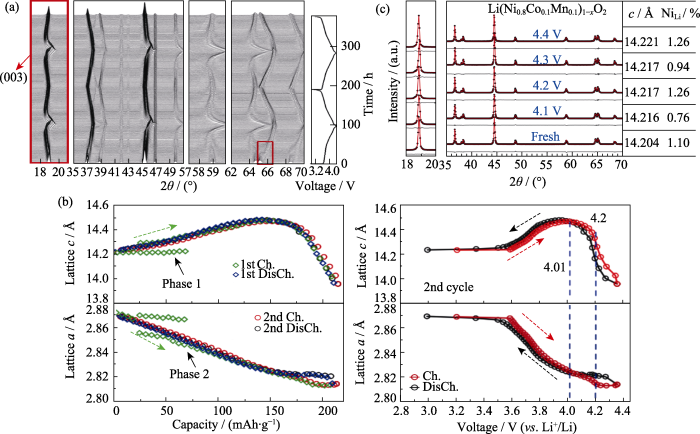
对于锂离子电池层状正极材料在循环过程中结构稳定性的研究, 一般认为, 在深度脱锂, 即高电压下, c轴的剧烈变化会导致循环过程中活性粒子开裂、颗粒间出现裂纹以及宏观应变, 这可能是高电压下电池循环性能较差的原因之一[45⇓⇓-48]。Li等[49]通过原位XRD及Rietveld精修研究了高镍层状正极材料LiNi0.8Co0.1Mn0.1O2 (NCM811)的衰减机理, 首先对NCM811/Li半电池进行了原位XRD表征, 如图8(a)所示, NCM811的(003)峰在首次充电时先向低角度移动, 而后向高角度移动, 表明锂层间距先增大后减小, 放电过程则相反。在第二个充放电过程中衍射峰位的变化是可逆的。图8(b)为充放电过程中晶胞参数随容量及电压的变化规律, 在整个充电过程中, 随着电压增大, 晶胞参数a逐渐减小, c则先增大后减小。值得注意的是晶胞参数随电压的变化是可逆的, 表明在充放电循环过程中晶胞沿c轴出现了反复的膨胀与收缩。同时, 他们对比了原始材料及循环后材料在不同充电状态下的晶胞参数变化, 研究了NCM811/石墨软包电池在循环200个周期后正极材料的晶体结构特征, 如图8(c)所示。XRD精修结果表明, 几种状态下的晶胞参数c接近, “Li/Ni反位”也没有明显差异, 说明高镍层状正极材料在充放电过程中的晶体结构变化较小, 这可能不是导致高电压下循环性能变差的主要原因。
图8
图8
高镍正极LiNi0.8Co0.1Mn0.1O2的充放电原位XRD表征结果[49]
Fig. 8
In situ XRD characterization of Ni-rich cathode LiNi0.8Co0.1Mn0.1O2(NCM811) during charge-discharge (© 2015, ECS)[49]
(a) In-situ XRD patterns of NCM811 cycled between 3.0-4.4 V at a rate of C/100 for two cycles; (b) Cell parameters c and a as functions of specific capacity and cell potential; (c) XRD refinement patterns, corresponding cell parameters and Li/Ni occupation information of fresh NCM811 electrode and the recovered electrodes that cycled 200 times to 4.1, 4.2, 4.3 and 4.4 V, respectively. 1 Å=0.1 nm; Ch: Charged; DisCh: Discharged. Colorful figures are available on website
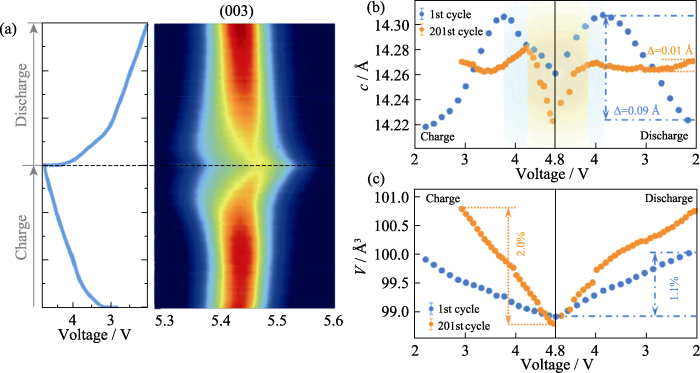
富锂层状正极材料的结构退化机理同样受到了研究者的广泛关注[50⇓⇓-53]。Wang等[54]通过原位同步辐射XRD研究了富锂层状正极材料在首次及第201次充放电过程中的结构变化规律, 图9(a)为Li1.2Ni0.13Co0.13Mn0.54O2正极材料第201次充放电原位XRD图谱, XRD数据的精修结果中(003)峰在充电初期逐渐向较低的角度偏移, 这说明锂离子脱出后过渡金属层之间的斥力增大导致晶胞参数c增大(图9(b))。然而, 值得注意的是, 这一过程在201次充电中被推迟到更高的电压(图9(b)), 这主要是由于在不断地充放电过程中 “Li/Ni反位” 增加, 引起了更高的锂离子扩散势垒。此外, 第201次充放电过程与首次充放电过程相比, 晶胞参数c变化较小, 晶胞体积变化(2.0%)比首次充放电(1.1%)增加了近两倍。较大的体积变化可能会进一步加剧材料内部的应力, 导致循环性能变差。另外作者结合X射线吸收近边谱(XANES)结果分析表明, 富锂层状正极材料的电化学衰减主要是由不同过渡金属之间的异步反应和化学-力学不稳定性所导致。
图9
图9
Li1.2Ni0.13Co0.13Mn0.54O2的充放电原位同步辐射XRD表征结果[54]
Fig. 9
In-situ XRD characterization of Li1.2Ni0.13Co0.13Mn0.54O2 during charge-discharge process (© 2021, Springer Nature)[54]
(a) Charge-discharge curve and corresponding contour plot of XRD pattern during the 201st cycle with the colour red to blue representing the decreasing peak intensity; (b, c) Lattice parameters (c and V) as functions of potential during the 1st and 201st cycles after the electrode activated at a rate of 0.1C. 1 Å=0.1 nm. Colorful figures are available on website
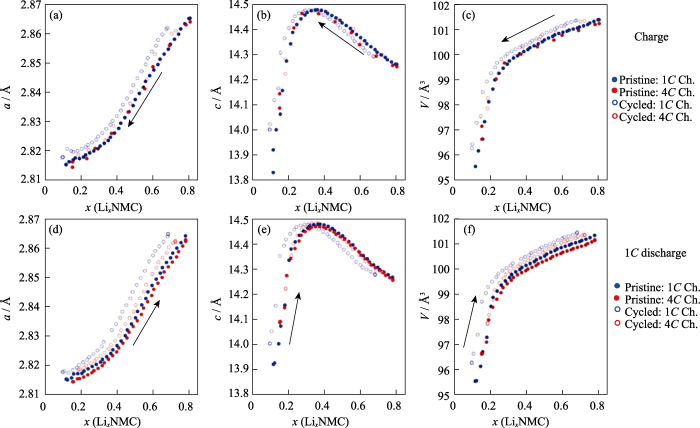
在锂离子电池的实际应用中, 快速充电性能也是备受关注的重要指标之一。当设计高功率应用的电池材料时, 电极材料的快速充放电过程可能与小倍率时的情况有很大不同, 因此, 深入理解其结构演变过程至关重要[55-56]。而具有数据采集快、亮度高特点的同步辐射XRD是研究电极材料在大电流密度下快速动态特性的重要表征手段。Quilty等[57]利用原位同步辐射XRD研究了NCM811/石墨电池中NCM811正极在不同充电倍率下的结构退化机理。他们首先对比了NCM811正极在充电倍率为1C和4C下的结构变化规律(1C=190 mA·g−1)。分别选取四个不同状态的电池: 不同的循环条件(初始与150次循环后)和充电倍率(1C与4C)。图10为XRD精修后的晶胞参数(a, c, V)随充放电的变化规律, 晶胞参数a和c的变化趋势没有明显区别。但值得注意的是, 无论是初始电极还是150次循环后的电极, 1C充电时的晶胞体积变化相比4C更大, 循环过程中体积变化更大可能导致正极材料颗粒破碎, 阻抗增大以及产生新的界面副反应, 从而造成容量衰减。
图10
图10
LiNi0.8Co0.1Mn0.1O2正极材料的充放电原位XRD Rietveld精修结果[57]
Fig. 10
In-situ XRD Rietveld refinement results of LiNi0.8Co0.1Mn0.1O2 cathode materials during charge and discharge process (© 2022, ECS)[57]
(a, d) Lattice parameters a; (b, e) Lattice parameters c; (c, f) Unit cell volumes. 1 Å=0.1 nm. Colorful figures are available on website
3.4 在正极材料结构改性中的应用
在锂离子电池正极材料的结构改性研究中, 通过XRD精修可以确定晶胞参数、原子坐标等信息, 特别是对于掺杂改性, 可以明确掺杂原子占位信息, 以及掺杂后晶体结构的变化情况, 进而阐明材料性能改善机理。
在对聚阴离子型正极材料LiMPO4 (M=Fe, Mn)的改性研究中, 一般采用掺杂等方式改善结构稳定性和电化学性能。Yang等[62] 合成了LiFePO4和LiFe0.95M0.05PO4(M=Mg2+, Ni2+, Al3+, V3+), 通过XRD Rietveld精修研究了阳离子掺杂对LiFePO4结构特征的影响规律, 进而明确了电化学性能改善的机理, 结果如表2所示。掺杂原子进入了Fe位, 掺杂元素较大的离子半径诱导Li-O键增长, 导致键能降低, 促进了锂离子传输, 改善了电化学性能。其中LiFe0.95V0.05PO4的Li-O键最长, 表现出最优的电化学性能, 在1C 电流密度下放电容量达到136 mAh·g−1。
表2 LiFePO4和LiFe0.95M0.05PO4的结构精修结果[62]
Table 2
| Sample | Doped atomic radius/nm | Displace ion radius/nm | Lattice constant/nm | Lattice volume/nm3 | Interatomic distance/nm | Reliability factor |
|---|---|---|---|---|---|---|
| LFP | rFe = 0.172 | rFe2+ = 0.074 | a=1.031634 b=0.600129 c=0.469139 | 0.29045 | Li-O1:0.21664 Li-O2:0.20901 Li-O3:0.21651 Li-O:0.214050 | Rwp = 7.72% Rp =5.63% χ2 = 2.794 |
| LFMgP | rMg = 0.172 | rMg2+ = 0.065 | a=1.031583 b=0.600035 c=0.469090 | 0.29036 | Li-O1:0.21712 Li-O2:0.21041 Li-O3:0.21665 Li-O:0.214720 | Rwp = 9.32% Rp = 6.79% χ2 = 2.878 |
| LFAlP | rAl = 0.182 | rAl3+ = 0.050 | a=1.032204 b=0.600358 c=0.469072 | 0.29068 | Li-O1:0.21670 Li-O2:0.21001 Li-O3:0.21747 Li-O:0.214730 | Rwp = 9.14% Rp = 6.60% χ2 = 2.989 |
| LFNiP | rNi = 0.162 | rNi2+ = 0.072 | a=1.031083 b=0.599820 c=0.468923 | 0.29001 | Li-O1:0.21734 Li-O2:0.20863 Li-O3:0.21586 Li-O:0.213950 | Rwp = 8.26% Rp = 6.12% χ2= 2.929 |
| LFVP | rV = 0.192 | rV3+ = 0.074 | a=1.032223 b=0.600494 c=0.469485 | 0.291 | Li-O1:0.21864 Li-O2:0.21074 Li-O3:0.21794 Li-O:0.215770 | Rwp = 9.86% Rp = 7.15% χ2= 2.426 |
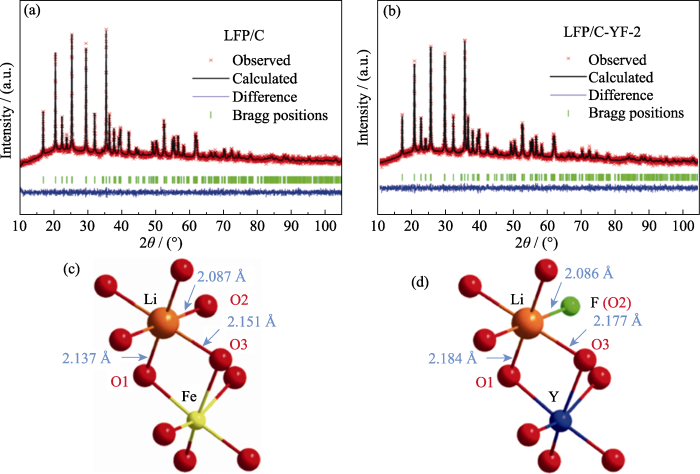
类似地, 通过XRD Rietveld精修确定阴阳离子协同掺杂时的聚阴离子型正极材料晶体结构信息也较为普遍。最近,Wang等[63]研究了Y,F协同掺杂的LiFePO4正极材料的结构信息及电化学性能, 分别对LiFePO4/C (LFP/C)、LiFe0.995Y0.005PO3.996F0.004/C (LFP/C-YF-1)、LiFe0.994Y0.006PO3.991F0.009/C (LFP/C-YF-2)和LiFe0.988Y0.012PO3.99F0.01/C (LFP/C-YF-3)正极材料进行XRD精修, 如图11(a, b)所示。XRD精修结果见表3, 掺杂后样品的晶胞参数a、b和c均变小,从而导致整个晶胞体积缩小。F-占据O2-位, Y3+占据Fe2+位, F-离子的半径(0.133 nm)小于O2-(0.14 nm), 导致单位晶胞体积缩小, 而Y3+的半径(0.09 nm)大于Fe2+(0.078 nm), 会导致单位晶胞体积膨胀。因此,整个晶胞的体积收缩意味F已经掺入到LiFePO4的晶格中。此外, Y3+占据Fe2+位使得结构中形成更多的Li空位,进而改善锂离子扩散能力。更值得注意的是,如图11(c, d)所示,与未掺杂的材料相比,掺杂后材料的Li-O键增长而P-O键缩短。Li-O增长使Li离子更容易嵌入和脱出晶格,进而表现出优异的高倍率充放电能力, LFP/C-YF-2正极材料在10C电流密度(1C=170 mA·g-1)下, 放电比容量可以达到135.8 mAh·g-1。
图11
图11
LiFePO4改性前后XRD精修结果[63]
Fig. 11
XRD Rietveld refinement results of LiFePO4 before and after modification (© 2021, RSC)[63]
(a, b) XRD Rietveld refinement patterns of (a) LFP/C and (b) LFP/C-YF-2; (c, d) Schematic diagrams of change in Li-O bond length of (c) LFP/C and (d) LFP/C-YF-2. 1 Å=0.1 nm. Colorful figures are available on website
表3 XRD精修得到LiFePO4改性前后的晶胞参数[63]
Table 3
| Sample | a/nm | b/nm | c/nm | V/nm3 |
|---|---|---|---|---|
| LFP/C | 1.03229 | 0.60061 | 0.46941 | 0.29104 |
| LFP/C-YF-1 | 1.03054 | 0.59985 | 0.46903 | 0.28994 |
| LFP/C-YF-2 | 1.03082 | 0.59977 | 0.46874 | 0.28980 |
| LFP/C-YF-3 | 1.03069 | 0.59989 | 0.46892 | 0.28994 |
表4 Al、Ti、Mg共掺杂LiCoO2及纯LiCoO2的XRD结构精修结果[64]
Table 4
| Atom | Site | x | y | z | Occupancy | Uiso |
|---|---|---|---|---|---|---|
| Lia | 3a | 0 | 0 | 0 | 1.000 | 0.014(6) |
| Coa | 3b | 0 | 0 | 0.50000 | 1.000 | 0.023(8) |
| Oa | 6c | 0 | 0 | 0.2300(6) | 1.000 | 0.049(1) |
| Lib | 3a | 0 | 0 | 0 | 0.98(1) | 0.020(1) |
| Mgb | 3a | 0 | 0 | 0 | 0.01(9) | 0.020(1) |
| Cob | 3b | 0 | 0 | 0.50000 | 0.99(7) | 0.001(2) |
| Alb | 3b | 0 | 0 | 0.50000 | 0.002(0) | 0.001(2) |
| Tib | 3b | 0 | 0 | 0.50000 | 0.001(0) | 0.001(2) |
| Ob | 6c | 0 | 0 | 0.2476(3) | 1.000 | 0.068(5) |
a: Bare LiCoO2 (Rwp=1.31%, Rp=0.86%, χ2=1.231); LiCoO2 (space group: R-3m); Lattice parameters: a=b=0.28158(5) nm, c=1.40513(2) nm, α=β=90°, γ=120°; Volume = 0.0964869 nm3
b: Al, Ti, Mg co-doped LiCoO2 (Rwp=3.37%, Rp=1.70%, χ2=1.023); LiCoO2 (space group: R-3m); Lattice parameters: a=b=0.28166(3) nm, c=1.40560(3) nm, α=β=90°, γ=120°; Volume=0.0965720 nm3
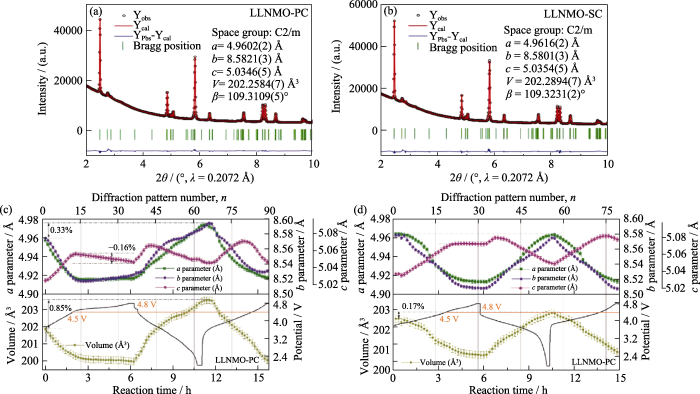
近年来, 单晶层状正极材料引起了研究者的极大兴趣, 与多晶材料相比, 其主要优点在于优异的锂离子传输性能及结构稳定性[65], 充分关注充放电过程中的晶体结构变化规律对于阐明单晶材料在电化学性能的优势必不可少。Yang等[66]借助原位同步辐射XRD及精修讨论了单晶与多晶富锂层状正极材料Li(Li0.2Ni0.2Mn0.6)O2 (LLNMO)的结构稳定性, 通过精修获得了单晶(LLNMO-SC)和多晶材料(LLNMO-PC)的晶体结构信息, 如图12(a, b)所示, 两种材料的晶体结构均为C2/m空间群, 且具有相似的晶胞参数。充放电过程中晶胞参数的变化规律如图12(c, d)所示, 结果表明在第一次充电至4.5 V的过程中, LLNMO-PC与LLNMO-SC的晶胞参数具有相同的变化规律, 但充电至4.8 V的过程中, LLNMO-PC的晶胞参数c变化较明显, 而LLNMO-SC几乎保持恒定, 证明了单晶材料在4.5~ 4.8 V范围内的结构稳定性。值得一提的是, 在第一次循环前后, LLNMO-SC的晶胞参数a、b、c和晶胞体积V的变化分别为0.00012、0.00039、0.00104 nm和0.0003441 nm3, 远远小于LLNMO-PC的变化(0.00112、0.00189、0.00221 nm和0.0014018 nm3), 有力地证明了单晶材料在循环过程中具有优异的结构可逆性和稳定性。
图12
图12
Li(Li0.2Ni0.2Mn0.6)O2正极材料的XRD结构精修结果[66]
Fig. 12
XRD Rietveld refinement results of Li(Li0.2Ni0.2Mn0.6)O2 cathode materials (© 2022, Wiley-VCH)[66]
(a, b) Experimental XRD patterns and Rietveld refinement results of (a) LLNMO-PC and (b) LLNMO-SC; (c, d) Changes of lattice parameters (a, b, c, and V) for (c) LLNMO-PC and (d) LLNMO-SC electrodes during charge and discharge 1 Å=0.1 nm. Colorful figures are available on website
高镍层状正极材料中“Li/Ni反位”导致锂离子扩散困难, 原子掺杂是抑制“Li/Ni反位”的常见改性手段。而XRD精修可以精确计算高镍层状正极材料“Li/Ni反位”占比。最近, Tan等[67]利用XRD精修证明了Pr掺杂对高镍层状正极材料LiNi0.9Co0.05Mn0.05O2中“Li/Ni反位”的抑制作用,结果表明Pr占据了晶格中的Ni位, 通过计算不同Pr掺杂量的材料中“Li/Ni反位”比例, 发现随着Pr掺杂量增大, “Li/Ni反位”比例降低, 证明Pr掺杂有利于抑制“Li/Ni反位”, 进而改善材料的锂离子扩散能力。由于“Li/Ni反位”比例较低, 掺杂后材料在循环过程中的结构稳定性得到了明显改善, 充放电100圈后容量保持率从78.7%提升至90.8%。另外, Zhang等[68]同样通过XRD精修证明Mg与Al共同掺杂对高镍层状正极材料LiNi0.95Co0.03Al0.01Mg0.01O2中的“Li/Ni反位”有明显抑制作用。Mg, Al共掺杂高镍层状正极材料的“Li/Ni反位”占6.49%, 而仅Mg掺杂、未掺杂的高镍层状正极材料中“Li/Ni反位”占比分别为7.39%、9.12%。“Li/Ni反位”占比较低, 使材料具有优异的大倍率放电能力, 在10C (1C=180 mA·g−1)电流密度下放电容量可以达到172.9 mAh·g−1。
在尖晶石型正极材料LiMn2O4的研究中, 许多研究者聚焦于通过掺杂的方式解决Jahn-Teller效应以改善材料的电化学性能, 并借助XRD结构精修揭示掺杂原子在稳定结构方面的作用。Cai等[69]研究了Al掺杂的LiMn2O4结构特征。对合成的样品LiMn2-xAlxO4 (x=0.05, 0.10, 0.16)进行XRD结构精修, 结果如表5所示。随着Al掺杂量增大, 晶胞体积随之变小, Al3+占据尖晶石结构八面体中Mn3+的16d位置。在尖晶石结构中晶胞体积较小, 使锂离子传输路径缩短, 进而改善了锂离子的扩散行为。另外, 随着Al3+占据Mn3+位, 晶胞中Mn3+的占比降低, 缓解了Jahn-Teller效应, 从而改善了LiMn2O4的电化学性能。
表5 Al掺杂LiMn2O4的XRD结构精修结果[69]
Table 5
| Formula | Calculated | Experimental | ||||
|---|---|---|---|---|---|---|
| a/nm | b/nm | c/nm | V/nm3 | a/nm | V/nm3 | |
| Li8Mn16O32 | 0.886205 | 0.886205 | 0.886205 | 0.695990 | - | - |
| Li8Mn15AlO32 | 0.826725 | 0.826725 | 0.826725 | 0.567617 | 0.82507 | 0.561658 |
| Li8Mn14Al2O32 | 0.831493 | 0.831493 | 0.799071 | 0.552416 | 0.82466 | 0.560821 |
| Li8Mn13Al3O32 | 0.814375 | 0.826337 | 0.820583 | 0.551780 | 0.82110 | 0.553590 |
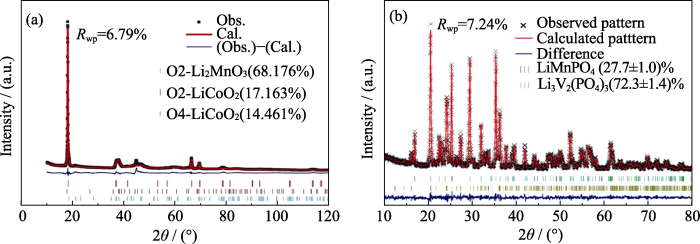
复相正极材料兼具各相正极材料的优势, XRD结构精修不仅可以明确相组成、晶胞参数等结构信息, 还可以确定复相正极材料的相比例, 以阐明复相材料中各个物相在电化学性能改善方面发挥的作用。Zuo等[70]通过XRD精修计算了所合成的富锂锰基正极材料Li1.25Co0.25Mn0.50O2, XRD精修图谱如图13(a)所示。结果表明, 所制备的材料由O2型Li2MnO3、O2型LiCoO2和O4型LiCoO2组成, 各相质量分数分别为68.176%、17.163%和14.461%, 表明成功合成了含有O2结构型富锂锰基正极材料, 具有这种结构的正极材料展现出优异的电化学性能, 放电比容量达到400 mAh·g−1。同样地, Cao等[71]通过XRD Rietveld 精修确定了锂离子电池复相正极材料LiMnPO4·Li3V2(PO4)3/C (LMVP/C)的晶体结构和相含量, 如图13(b)。LiMnPO4 (LMP)相和Li3V2(PO4)3 (LVP)相的Rietveld 精修分别基于正交(空间群Pb nm)和单斜晶(空间群P21/n)晶体结构。对比LMVP/C与单相LMP及LVP的晶胞体积变化, 结果表明LMVP的晶胞体积大于LMP, 而小于LVP, 因此, 可以认为LMVP中存在LMP与LVP互相掺杂的现象, 这有利于提高其电导率及锂离子扩散系数。同时通过Rietveld精修计算了LMP和LVP的质量分数分别为(27.7±1.0)%和(72.3±1.4)%。近年来, 关于通过XRD Rietveld精修确定层状/尖晶石复相正极材料结构特征的报道也较为常见。Lu等[72]研究了Li0.87Mn0.80O1.81F0.19 (LMOF)正极材料的结构特征。XRD Rietveld精修结果表明, LMOF是由层状相(R-3m)和尖晶石相(Fd-3m)两相组成, 其相含量分别为86.56%和13.44%。此外, 精修结果表明F占据O位, 形成较强的Mn-F键, 抑制了Mn向Li空位迁移, 进而稳定层状结构。
图13
4 结论与展望
综上所述, 锂离子电池正极材料的结构不同, 导致锂的配位环境也出现差别, 进而表现出不同的化学稳定性及锂离子传输特征, XRD Rietveld结构精修是研究正极材料结构信息的强有力工具。本文首先介绍了XRD 技术及Rietveld精修策略, 然后围绕几类典型正极材料, 重点讨论了XRD Rietveld精修在正极材料合成、退化衰减和结构改性中的应用。XRD Rietveld精修可以揭示正极材料在合成过程中的结构演变规律, 为有序结构的定向合成提供指导, 以及可以明确正极材料在退化与衰减过程中的晶胞参数变化, 深入理解衰减机理, 进一步指导正极材料的结构改性, 对建立材料结构特征与性能间的“构-效”关系具有重要意义。
在未来科学研究中, 发展高能X射线技术对正极材料的结构研究意义重大, 如X射线自由电子激光(XFEL), XFEL产生的飞秒X射线使其表现出纳米尺度空间分辨率和飞秒尺度时间分辨率, 有益于对锂电正极材料的动态行为进行快速时间分辨研究, 结合Rietveld精修, 可以深入研究非平衡状态下的结构演变[73], 如精准跟踪化学/电化学过程中亚稳态中间相的形成, 快速充电下的动态失效模式等实际问题, 这将为正极材料退化和失效以及正极材料的定向合成机制提供清晰的见解。然而, XRD技术一般反馈正极材料内部平均结构信息, 对于局部精细结构信息的解析略显不足, 特别是对于轻元素(Li、O)的识别能力有限, 以及无法区分相邻原子序数元素(如Ni、Co、Mn)。因此还需要和其他多种技术手段相结合来深入理解正极材料的精细结构。利用光子与物质相互作用的原理对正极材料的结构表征除了常见的X射线衍射外, 还有用于分析局域结构的扩展X射线吸收精细谱、识别晶格畸变的拉曼光谱等。当然, 除了利用光子与物质的相互作用外, 还有利用电子与物质的相互作用, 如电子衍射, 分析纳米尺度正极材料局部原子排列;利用中子与物质相互作用的中子衍射, 精确区分邻近原子及Li等轻原子的占位信息。未来发展高能XRD Rietveld精修与其他结构表征手段的高度有机结合, 对推动锂离子电池正极材料的结构解析及发展具有重要意义。
参考文献
Issues and challenges facing rechargeable lithium batteries
Energy materials in new era.
In the long river of human history, every technological revolution is accompanied by transition of cognition, development and utilization of energy. At present, China has become the No. 1 in the world in both production and consumption of energy, which continue rising in the excepted future. Developing energy technology is still a key way to solve the problems of excessive dependence on traditional fossil energy and environmental pollution, construct a reasonable social structure, promote the sustainable development of human society, and achieve the goals of carbon emission peaking and carbon neutrality. In 2020, renewable energy in China such as photovoltaics and wind power evolved marvelously which occupied 1/3 of global total volume. In this regard, energy materials are indispensable components, which play the core role in realizing conversion and utilization of clean energy, developing new energy technologies, and supporting the entire energy system.<br>In recent years, energy materials have achieved extensive and sustainable development in many fields, including secondary batteries, fuel cells, solar cells, supercapacitors, photoelectric catalysis, and energy-containing materials. For example, high nickel ternary materials as cathode material in the lithium-ion battery are leading the future of a new generation of automotive power battery technology towards faster charging speeds, longer service life and longer mileage<sup>[1-4]</sup>. The ever increasing demand for energy storage has also spawned simultaneously a series of new battery technologies, such as lithium-sulfur<sup>[5]</sup>, lithium-air<sup>[6]</sup> and solid-state batteries<sup>[7]</sup>. They have advantages in energy density, economy and safety, but technical defects (<em>e.g.</em>, shuttle effect in Li-S battery attributed to polysulfides, blockage of matrix pores in Li-air battery attributed to discharging product, unsatisfactory electrical conductivity of electrolyte in solid-state battery) are frustrating. Technological improvement and industrialization are strongly dependent on the innovative design and structural optimization of electrode and electrolyte materials. To promote the share of renewable energy in primary source, photovoltaics, the representative of new energy, received great expectation. In addition, halogen perovskite-based third-generation solar cell technology has achieved a solar energy conversion efficiency comparable to that of silicon single crystal, showing a prosperous photovoltaic industry in the future<sup>[8]</sup>. However, its sensitivity to temperature, humidity, light, and oxygen<sup>[9]</sup>, and inevitable Pb-containing raw material in preparation still need to find a solution in the underlying materials design. Moreover, as continuously optimizing the traditional catalyst materials, like Pt and Pd, as well as the non-precious and non-metallic catalysts, the energy conversion efficiency of fuel cells has been gradually improved with reduction of their technical costs, meeting a certain degree of commercial application<sup>[10-11]</sup>. Besides, photocatalytic and electrocatalytic technologies for CO<sub>2</sub> reduction and nitrogen fixation also provide a new way for the storage and utilization of renewable energy, technically support the carbon emission peak in 2030 and carbon neutrality in 2060<sup>[12-13]</sup>. <br>In the context of the era of sustainable development and the fiercely competitive international scientific and technological frontier research environment, in the energy materials research, including the exploration of physical and chemical properties, functional discovery, precise design and preparation of nanomaterials, and advanced device assembly, China has made many important breakthroughs. In order to focus on displaying the research results of Chinese scholars in this field, to promote academic exchanges among peers, and to stimulate interest in energy materials from all walks of life, Nanjing University of Science and Technology, Shanghai Institute of Ceramics, Huazhong University of Science and Technology, <em>etc.</em> hereby organize the publication of “Energy Materials Special Issue”, containing the latest research articles and reviews related to energy materials involved with perovskite photovoltaics, semitransparent solar cell, Li-ion battery, Mg battery, Li-S battery, thermoelectrics, CO<sub>2</sub> splitting, <em>etc</em>. It is hoped that this Special Issue can offer useful references for the scientific research and disciplinary development of energy materials in China.
Building practical high-voltage cathode materials for lithium-ion batteries
Insights for understanding multiscale degradation of LiFePO4 cathodes
Fundamental understanding and practical challenges of anionic redox activity in Li-ion batteries
A review on cathode materials for advanced lithium ion batteries: microstructure designs and performance regulations
Synchrotron radiation based X-ray techniques for analysis of cathodes in Li rechargeable batteries
Synchrotron X-rays are able to depict the information of structural order, oxidation state, atomic structure, chemical phase mapping and pores within cathode materials of Li-rechargeable batteries.
Line profiles of neutron powder-diffraction peaks for structure refinement
The structure of some crystals as indicated by their diffraction of X-rays
In situ X-ray diffraction techniques as a powerful tool to study battery electrode materials
Features and futures of X-ray free-electron lasers
Accelerator-based X-ray sources: synchrotron radiation, X-ray free electron lasers and beyond
Anomalous Bragg peak widths in LixTiS2
Lab-scale in situ X-ray diffraction technique for different battery systems: designs, applications, and perspectives
Using in-situ laboratory and synchrotron-based X-ray diffraction for lithium-ion batteries characterization: a review on recent developments
Renewable technologies, and in particular the electric vehicle revolution, have generated tremendous pressure for the improvement of lithium ion battery performance. To meet the increasingly high market demand, challenges include improving the energy density, extending cycle life and enhancing safety. In order to address these issues, a deep understanding of both the physical and chemical changes of battery materials under working conditions is crucial for linking degradation processes to their origins in material properties and their electrochemical signatures. In situ and operando synchrotron-based X-ray techniques provide powerful tools for battery materials research, allowing a deep understanding of structural evolution, redox processes and transport properties during cycling. In this review, in situ synchrotron-based X-ray diffraction methods are discussed in detail with an emphasis on recent advancements in improving the spatial and temporal resolution. The experimental approaches reviewed here include cell designs and materials, as well as beamline experimental setup details. Finally, future challenges and opportunities for battery technologies are discussed.
Deciphering the thermal behavior of lithium rich cathode material by in situ X-ray diffraction technique
Rietveld refinement guidelines
A set of general guidelines for structure refinement using the Rietveld (whole-profile) method has been formulated by the International Union of Crystallography Commission on Powder Diffraction. The practical rather than the theoretical aspects of each step in a typical Rietveld refinement are discussed with a view to guiding newcomers in the field. The focus is on X-ray powder diffraction data collected on a laboratory instrument, but features specific to data from neutron (both constant-wavelength and time-of-flight) and synchrotron radiation sources are also addressed. The topics covered include (i) data collection, (ii) background contribution, (iii) peak-shape function, (iv) refinement of profile parameters, (v) Fourier analysis with powder diffraction data, (vi) refinement of structural parameters, (vii) use of geometric restraints, (viii) calculation of e.s.d.'s, (ix) interpretation ofRvalues and (x) some common problems and possible solutions.
AC impedance analysis of polycrystalline insertion electrodes: application to Li1-xCoO2
Local structure and electronic structure of LiFePO4 as a cathode for lithium-ion batteries
Spinel LiMn2O4 cathode materials in wide voltage window: single-crystalline versus polycrystalline
Single-crystal (SC) layered oxides as cathodes for Li-ion batteries have demonstrated better cycle stability than their polycrystalline (PC) counterparts due to the restrained intergranular cracking formation. However, there are rare reports on comparisons between single-crystal LiMn2O4 (SC-LMO) and polycrystalline LiMn2O4 (PC-LMO) spinel cathodes for Li-ion storage. In this work, the Li-ion storage properties of spinel LiMn2O4 single-crystalline and polycrystalline with similar particle sizes were investigated in a wide voltage window of 2–4.8 V vs. Li/Li+. The SC-LMO cathode exhibited a specific discharge capacity of 178 mA·h·g−1, which was a bit larger than that of the PC-LMO cathode. This is mainly because the SC-LMO cathode showed much higher specific capacity in the 3 V region (Li-ion storage at octahedral sites with cubic to tetragonal phase transition) than the PC-LMO cathode. However, unlike layered-oxide cathodes, the PC-LMO cathode displayed better cycle stability than the SC-LMO cathode. Our studies for the first time demonstrate that the phase transition-induced Mn(II) ion dissolution in the 3 V region rather than cracking formation is the limiting factor for the cycle performance of spinel LiMn2O4 in the wide voltage window.
A review of nickel-rich layered oxide cathodes: synthetic strategies, structural characteristics, failure mechanism, improvement approaches and prospects
Challenges and strategies of lithium-rich layered oxides for Li-ion batteries
Phospho-olivines as positive-electrode materials for rechargeable lithium batteries
Review of spinel LiMn2O4 cathode materials under high cut-off voltage in lithium-ion batteries: challenges and strategies
Goodenough
Intrinsic kinetic limitations in substituted lithium-layered transition-metal oxide electrodes
Substituted Li-layered transition-metal oxide (LTMO) electrodes such as LiNiMnCoO (NMC) and LiNiCoAlO (NCA) show reduced first cycle Coulombic efficiency (90-87% under standard cycling conditions) in comparison with the archetypal LiCoO (LCO; ∼98% efficiency). Focusing on LiNiCoAlO as a model compound, we use operando synchrotron X-ray diffraction (XRD) and nuclear magnetic resonance (NMR) spectroscopy to demonstrate that the apparent first-cycle capacity loss is a kinetic effect linked to limited Li mobility at > 0.88, with near full capacity recovered during a potentiostatic hold following the galvanostatic charge-discharge cycle. This kinetic capacity loss, unlike many capacity losses in LTMOs, is independent of the cutoff voltage during delithiation and it is a reversible process. The kinetic limitation manifests not only as the kinetic capacity loss during discharge but as a subtle bimodal compositional distribution early in charge and, also, a dramatic increase of the charge-discharge voltage hysteresis at > 0.88. Li NMR measurements indicate that the kinetic limitation reflects limited Li transport at > 0.86. Electrochemical measurements on a wider range of LTMOs including Li(Ni,Fe)CoO suggest that 5% substitution is sufficient to induce the kinetic limitation and that the effect is not limited to Ni substitution. We outline how, in addition to a reduction in the number of Li vacancies and shrinkage of the Li-layer size, the intrinsic charge storage mechanism (two-phase vs solid-solution) and localization of charge give rise to additional kinetic barriers in NCA and nonmetallic LTMOs in general.
Recent breakthroughs and perspectives of high-energy layered oxide cathode materials for lithium ion batteries
High-energy Ni-rich Li[NixCoyMn1-x-y]O2 cathodes via compositional partitioning for next-generation electric vehicles
Crack-free single-crystalline Co-free Ni-rich LiNi0.95Mn0.05O2 layered cathode
Probing thermally- induced structural evolution during the synthesis of layered Li-, Na-, or K-containing 3D transition-metal oxides
Reaction mechanisms of layered lithium-rich cathode materials for high-energy lithium-ion batteries
Nickel-rich layered cathode materials for lithium-ion batteries
Ni/Li disordering in layered transition metal oxide: electrochemical impact, origin, and control
Lithium ion batteries (LIBs) not only power most of today's hybrid electric vehicles (HEV) and electric vehicles (EV) but also are considered as a promising system for grid-level storage. Large-scale applications for LIBs require substantial improvement in energy density, cost, and lifetime. Layered lithium transition metal (TM) oxides, in particular, Li(NiMnCo)O (NMC, + + = 1) are the most promising candidates as cathode materials with the potential to increase energy densities and lifetime, reduce costs, and improve safety. In order to further boost Li storage capacity, a great deal of attention has been directed toward developing Ni-rich layered TM oxides. However, structural disorder as a result of Ni/Li exchange in octahedral sites becomes a critical issue when Ni content increases to high values, as it leads to a detrimental effect on Li diffusivity, cycling stability, first-cycle efficiency, and overall electrode performance. Increasing effort has been dedicated to improving the electrochemical performance of layered TM oxides via reduction of cationic mixing. Therefore, it is important to summarize this research field and provide in-depth insight into the impact of Ni/Li disordering on electrochemical characteristics in layered TM oxides and its origin to accelerate the future development of layered TM oxides with high performance. In this Account, we start by introducing the Ni/Li disordering in LiNiO, the experimental characterization of Ni/Li disordering, and analyzing the impact of Ni/Li disordering on electrochemical characteristics of layered TM oxides. The antisite Ni in the Li layer can limit the rate performance by impeding the Li ion transport. It will also degrade the cycling stability by inducing anisotropic stress in the bulk structure. Nevertheless, the antisite Ni ions do not always bring drawbacks to the electrochemical performance; some studies including our works found that it can improve the thermal stability and the cycling structure stability of Ni-rich NMC materials. We next discuss the driving forces and the kinetic advantages accounting for the Ni/Li exchange and conclude that the steric effect of cation size and the magnetic interactions between TM cations are the two main driving forces to promote the Ni/Li exchange during synthesis and the electrochemical cycling, and the low energy barrier of Ni migration from the 3a site in the TM layer to the 3b site in the Li layer further provides a kinetic advantage. Based on this understanding, we then review the progress made to control the Ni/Li disordering through three main ways: (i) suppressing the driving force from the steric effect by ion exchange; (ii) tuning the magnetic interaction by cationic substitution; (iii) kinetically controlling Ni migration. Finally, our brief outlook on the future development of layered TM oxides with controlled Ni/Li disordering is provided. It is believed that this Account will provide significant understanding and inspirations toward developing high-performance layered TM oxide cathodes.
In situ probing and synthetic control of cationic ordering in Ni-rich layered oxide cathodes
In situ XRD studies during synthesis of single-crystal LiNiO2, LiNi0.975Mg0.025O2, and LiNi0.95Al0.05O2 cathode materials
High-nickel, cobalt-free, single-crystal positive electrode materials could provide the ultimate intersection of high-specific capacity, low cost, and long-lifetime in lithium-ion batteries. In this work, the synthesis of LiNiO2, LiNi0.975Mg0.025O2, and LiNi0.95Al0.05O2 is studied by dynamic XRD during heating, in order to guide improvements in synthesis procedures. A comparison of Li2CO3 and LiOH·H2O lithium sources shows that either can be used to prepare these materials, but Li2CO3 requires a higher temperature. Mg doping is shown to be beneficial in lowering the temperature required to get fully lithiated, crystalline material. Additional experiments show that synthesis with a 480 °C preheat step, or synthesis directly from individual metal hydroxides (without a precursor), could be used as potentially viable alternative synthesis methods.
Kinetic control of long- range cationic ordering in the synthesis of layered Ni-rich oxides
Ultrafast solid-liquid intercalation enabled by targeted microwave energy delivery
Mechanism of targeted microwave energy transfer drives solid-liquid intercalation to completion in minutes.
Development of microstrain in aged lithium transition metal oxides
Bulk fatigue induced by surface reconstruction in layered Ni-rich cathodes for Li-ion batteries
Structural transformation of LiNi0.8Co0.1Mn0.1O2 cathode material during cycling with overcharge investigated by in situ X-ray diffraction
Phase transformation behavior and stability of LiNiO2 cathode material for Li-ion batteries obtained from in situ gas analysis and operando X-ray diffraction
Study of the failure mechanisms of LiNi0.8Mn0.1Co0.1O2 cathode material for lithium ion batteries
Building homogenous Li2TiO3 coating layer on primary particles to stabilize Li-rich Mn-based cathode materials
Origin of structural degradation in Li-rich layered oxide cathode
A novel strategy to significantly enhance the initial voltage and suppress voltage fading of a Li-and Mn-rich layered oxide cathode material for lithium-ion batteries
Structural features of complete and partial activation of Li-rich cathodes studied by in-situ XRD.
Reaction inhomogeneity coupling with metal rearrangement triggers electrochemical degradation in lithium-rich layered cathode
High-energy density lithium-rich layered oxides are among the most promising candidates for next-generation energy storage. Unfortunately, these materials suffer from severe electrochemical degradation that includes capacity loss and voltage decay during long-term cycling. Present research efforts are primarily focused on understanding voltage decay phenomena while origins for capacity degradation have been largely ignored. Here, we thoroughly investigate causes for electrochemical performance decline with an emphasis on capacity loss in the lithium-rich layered oxides, as well as reaction pathways and kinetics. Advanced synchrotron-based X-ray two-dimensional and three-dimensional imaging techniques are combined with spectroscopic and scattering techniques to spatially visualize the reactivity at multiple length-scales on lithium- and manganese-rich layered oxides. These methods provide direct evidence for inhomogeneous manganese reactivity and ionic nickel rearrangement. Coupling deactivated manganese with nickel migration provides sluggish reaction kinetics and induces serious structural instability in the material. Our findings provide new insights and further understanding of electrochemical degradation, which serve to facilitate cathode material design improvements.© 2021. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Extreme fast charge aging: correlation between electrode scale and heterogeneous degradation in Ni-rich layered cathodes
High-rate structure-gradient Ni- rich cathode material for lithium-ion batteries
Elucidating cathode degradation mechanisms in LiNi0.8Mn0.1Co0.1O2 (NMC811)/ graphite cells under fast charge rates using operando synchrotron characterization
Li-ion batteries capable of extreme fast charging (XFC) are in demand to facilitate widespread electric vehicle (EV) adoption. While the impact of fast charge on the negative electrode has been studied, degradation of state-of-the-art NMC811 under XFC conditions has not been studied in detail. Herein, cathode degradation is probed in NMC811/graphite batteries by analysis of structural and chemical changes for recovered samples previously cycled under XFC conditions and during typical cycling. NMC surface reconstruction, as determined by soft X-ray absorption, was not detected for recovered electrodes. However, bulk redox activity from X-ray absorption near edge structure measurements showed more change in the oxidation state of Ni and Co under the 1C charge rate compared to the 4C rate consistent with the electrochemistry. Increased unit cell volume contraction under the 1C rate as determined by operando X-ray diffraction suggests that higher charge rates may provide a protective effect on the cathode by reducing structural distortion due to less delithiation.
Revealing the degradation mechanism of Ni-rich cathode materials after ambient storage and related regeneration method
Origin of self- discharge mechanism in LiMn2O4-based Li-ion cells: a chemical and electrochemical approach
Significant improvement on electrochemical performance of LiMn2O4 at elevated temperature by atomic layer deposition of TiO2 nanocoating
Investigation of the self- discharge behaviors of the LiMn2O4 cathode at elevated temperatures: in situ X-ray diffraction analysis and a co-doping mitigation strategy
The doping effect on the electrochemical properties of LiFe0.95M0.05PO4 (M=Mg2+, Ni2+, Al3+, or V3+ ) as cathode materials for lithium-ion cells
Y-F co-doping behavior of LiFePO4/C nanocomposites for high-rate lithium-ion batteries
A novel bifunctional self- stabilized strategy enabling 4.6 V LiCoO2 with excellent long-term cyclability and high-rate capability
Kinetic limitations in single-crystal high-nickel cathodes
Structural origin of suppressed voltage decay in single-crystalline Li-rich layered Li[Li0.2Ni0.2Mn0.6]O2 Cathodes
Lattice engineering to refine particles and strengthen bonds of the LiNi0.9Co0.05Mn0.05O2 cathode toward efficient lithium ion storage
Gradient doping Mg and Al to stabilize Ni-rich cathode materials for rechargeable lithium-ion batteries
High electrochemical stability Al-doped spinel LiMn2O4 cathode material for Li-ion batteries
A high-capacity O2-type Li-rich cathode material with a single-layer Li2MnO3 superstructure
Nanorod-nanoflake interconnected LiMnPO4·Li3V2(PO4)3/C composite for high-rate and long-life lithium-ion batteries
Heavy fluorination via ion exchange achieves high-performance Li-Mn-O-F layered cathode for Li-ion batteries
X-ray imaging detectors for synchrotron and XFEL sources