|
|
New Applications of Solid State Ionics
WEN Zhao-Yin, LI Jing-Ze
2013 Vol. 28 (11): 1163–1164
 Abstract
Abstract(
1324 )
 HTML
HTML
 PDF
PDF(222KB)(
1355
)
During the past four decades, Solid State Ionics (SSI) field has attracted numerous researchers and engineers to cultivate new technologies which plays important roles in the development of sustainable energy utilization and clean transportation tools. The commercialization of lithium ion battery, sodium sulfur battery, sodium chloride battery, solid oxygen sensors have illustrated the success of SSI’s theories and technologies. The discovery and successful applications of ionic or mixed conductive materials, such as LiCoO2 and LiFePO4 as active materials of lithium ion battery cathode, Na-β/β″-Al2O3 ceramics as solid electrolytes and separators for sodium based batteries, ZrO2 as electrolytes for SOFC and oxygen sensors, and the newly developed Li10GeP2S12[1] and Li7La3Zr2O12[2] as lithium ion conductor, laid a solid foundation for the development of SSI technologies.
Recently, rechargeable lithium metal anode based batteries have become hot research topics in the field of SSI due to their extremely high specific capacity suitable for future generation of power sources in portable electronic devices, electric vehicles and energy storage systems. Among all the Li anode based batteries, Li-S and Li-air battery systems are the two most attractive candidates because of the low cost and abundance of sulfur and air as cathode active materials, especially the characteristics of easy access of active O2 from the surrounding air for Li-air batteries.
Breakthroughs have been made recently with these batteries. SION Power’s Li-S cells reached the highest practical specific energy as high as 350 Wh/kg, with a pack of 576 cells engineering the QinetiQ Zephyr Unmanned Aerial Vehicle (UAV) more than 336 h (14 d) of continuous flight, significantly surpassing the previous official record[3]. Their energy densities are higher than 500 Wh/kg for more than 500 cycles[3] and commercialization in the near future[4] are expected. Many Chinese institutions successfully prepared soft package lithium sulfur batteries[5]. The highest specific capacity of sulfur electrode over 900 mAh/g at 2C rate was realized by the Shanghai Institute of Ceramics, Chinese Academy of Sciences. Important advances in Li-air batteries were also made by designing carbon free air electrode [6,7] and by the aid of LATP solid lithium electrolyte lately[8]. However, the development of the rechargeable high energy lithium metal batteries are still hindered by the high reactivity of lithium metal with liquid electrolytes and the occurrence of dendrite growth during charge and discharge cycles. Moreover, the cycling stability of the batteries are still far away from the standard of practical applications of electric vehicle and electronic devices. Owing to the intrinsic highly resistive feature of the cathode materials, sulfur and lithium containing resultants with oxygen, structural and compositional designs of the cathode, the protection of lithium metal anode, and the control of the electrode/electrolyte interface are urgent matter to develop practical rechargeable lithium batteries.
SSI has been tackling the vital technical problems of high performance devices with solid ionic and mixed conductors as their key materials, which has great potential contribution to future energy and environmental needs of our society.
|
|
|
Recent Development of Aqueous Sodium Ion Batteries and Their Key Materials
YANG Han-Xi,QIAN Jiang-Feng
2013 Vol. 28 (11): 1165–1171
 Abstract
Abstract(
1939 )
 HTML
HTML
 PDF
PDF(383KB)(
1965
)
Sodium ion batteries are now actively developed as a new generation of electric energy storage technology because of their advantages of resource abundance and low cost. Particularly for large scale stationary electric storage, aqueous Na-ion batteries display better safety, lower cost and better environmental compatibility, providing a great prospect for widespread applications. However, there exist a number of difficulties in the development of Na-storage materials and aqueous Na-ion technology. To solve these problems, this review analyzes the different chemistry of the Na-storage materials and their electrochemical reactions in aqueous electrolytes, and discusses the recent development of Na+ host materials and aqueous Na-ion batteries. In addition, based on our experience and understanding, possible strategies are proposed for further development of low cost and pollution-free materials for aqueous Na-ion batteries.
|
|
|
Progress of Research on the Conductive Mechanism of the Glassy Electrolytes in Lithium Ion Batteries
ZHENG Yue-Lei, CHEN Ren-Jie, WU Feng, LI Li
2013 Vol. 28 (11): 1172–1180
 Abstract
Abstract(
1314 )
 HTML
HTML
 PDF
PDF(2330KB)(
2068
)
Glassy electrolytes have broad prospects for the application in all solid-state lithium ion batteries since they have isotropic conductivity and higher lithium ionic conductivity compared with ceramic electrolytes. In recent years, numerous groups have attempted to improve the lithium ionic conductivity, chemical and electrochemical stability of glassy electrolytes through nitrogen-incorporation by radio-frequency magnetron sputtering, preparing mixed former glasses and glass-ceramics by special technologies. In present review, conductive characteristics and mechanism in various glassy electrolytes are introduced. The micro-mechanisms of mixed former effect in several typical glassy electrolytes are discussed emphatically. The mixed former effect produces the non-bridge oxygen providing lithium-ion with the vacant place to move into or out, as well as expanding the lithium-ion conduction pathway to enhance ionic mobility in the network. The effect is brought about by the phase separation in micro structure of glassy electrolytes, which can be described as the isolation of continuous Li-rich phase with high ionic conductivity and isolated Li-poor phase with low ionic conductivity in micro structure. Finally, the premise conditions of mixed former effect occurring to a ternary glassy are summarized. Further study on conductive mechanisms of glassy electrolytes has important guiding significance for theory in developing glassy electrolytes with good chemical and electrochemical performances.
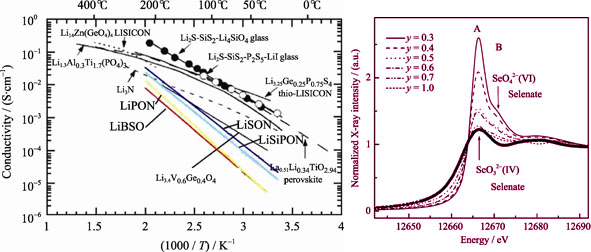
|
|
|
Current Status, Problems and Challenges in Lithium-sulfur Batteries
HU Jing-Jing, LI Guo-Ran, GAO Xue-Ping
2013 Vol. 28 (11): 1181–1186
 Abstract
Abstract(
2451 )
 HTML
HTML
 PDF
PDF(375KB)(
14233
)
Lithium-sulfur battery, fabricated with metal lithium as anode and sulfur as cathode, has received more attention as the most promising high energy power sources due to its high theoretical energy density (2600 Wh/kg). However, there are some serious and unavoidable problems for lithium-sulfur battery based on the dissolution-deposition processes in organic electrolyte, including serious structure change of metallic lithium anode, the lower utilization and poor cycle performance of active materials, which become a big barrier for the research and development of lithium-sulfur battery. The current status, problems and challenges of lithium-sulfur battery are summarized, including the sulfur-based cathode composites, electrolyte and lithium anode.
|
|
|
Nano-structure Effect on Solid State Fuel Cells Cathode Durability
HONG Tao, WANG Yao, XIA Chang-Rong
2013 Vol. 28 (11): 1187–1194
 Abstract
Abstract(
858 )
 HTML
HTML
 PDF
PDF(613KB)(
1451
)
Nano-structure could effectively enhance the electro-chemical properties of solid oxide fuel cell cathodes and the output power of single cells, which has a good application prospect. This article mainly focuses on the literatures reported about the long term stability of nano-structure cathode and electrode stability theoretical models. Nano-structure cathode has excellent stability at relatively low temperatures. Isothermal grain growth of nano-particles is self-limited because of the size effect. And nano-structure could significantly reduce the micro-strain induced by the misfit between electrolyte and electrocatalyst, which can keep the two phase in good contact. In addition, nano-structure can also weaken the interface break between two-phase particles in thermal cycle process. The performance of La0.8Sr0.2MnO3-δ and La0.6Sr0.4Co0.2Fe0.8O3-δ nano-structure cathodes increases by 2.3-78 times compared with traditional composites, and maintains a stable power output in 1000 h.
|
|
|
Electrochemical Performance of Silicon/Carbon/Graphite Composite Anode for Lithium Ion Batteries
PENG Peng, LIU Yu, WEN Zhao-Yin
2013 Vol. 28 (11): 1195–1199
 Abstract
Abstract(
1144 )
 HTML
HTML
 PDF
PDF(446KB)(
1694
)
Silicon and graphite with polyvinylidene fluoride as the matrix was pyrolyzed to obtain the Si/C/graphite composite anode with the stable and excellent electrochemical performance. The morphology of the composite was characterized by transmission electron microscope (TEM). The result showed that the composite had core-shell structure in which silicon particles were wrapped by the amorphous carbon. The effects of the silicon particle size and the graphite content on the electrode electrochemical performance were investigated systematically. It reveals that the fine size of silicon particles is beneficial to obtain a stable electrochemical performance. The appropriate content ratio of graphite and silicon is necessary to increase the reversible capacity of the composite. When the mass ratio of silicon to graphite is 1:1 and the silicon particle size is 50 nm, the Si/C/graphite composite presents the first discharge specific capacity of about 1741.6 mAh/g and the first coulombic efficiency of 72.4%. The reversible specific capacity still remains 820 mAh/g after 60 cycles. It demonstrates that the carbon-coating structure can be obtained by pyrolyzing the organic matter which will improve the electrochemical performance of the silicon-based anodes effectively.
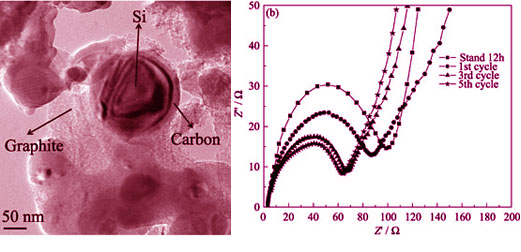
|
|
|
Effect of Mn2+ Doping on Structure and Electrochemical Properties of V2O5 as the Rechargeable Electrode Material
LI Zhao-Long, SUN Hua-Jun, XU Jie, ZHANG Xiao-Yan, HUANG Sheng-Nan, ZHU Quan-Yao, CHEN Wen, Galina S. Zakharova
2013 Vol. 28 (11): 1200–1206
 Abstract
Abstract(
764 )
 HTML
HTML
 PDF
PDF(637KB)(
1574
)
MnxV2O5 (x=0~0.06) was prepared by hydrothermal method with H2O2 and V2O5 as precursors and Mn2+ added directly in the sol preparation process. Structures and morphologies of the materials were characterized by X-ray diffraction (XRD), X-ray Photoelectron Spectroscope (XPS) and scanning electron microscope (SEM). The results indicate that the V2O5 is well crystallized as an orthorhombic structure without any detectable impurity phase with the doping of Mn2+. The doping Mn2+ is situated between anionic sheets of VO6 chain in the V2O5 and the morphology of V2O5 changes from nanorods to nanobelts cluster. The electrochemical behavior of the MnxV2O5 acted as cathode materials is studied by charge-discharge test and electrochemical impedance spectra (EIS) test through a three electrode system. The results indicate that the efficiency of the first charge-discharge of the material enhances to 90% with the Mn2+ doping. The discharge capacity of the Mn0.01V2O5 is up to 192.2 mAh/g after 90 charge-discharge cycles at 0.2C rate. The doping Mn2+ performs efficiently in ionic conductivity of V2O5 electrode material, and the ionic conductivity of Mn0.02V2O5 is enhanced from 6.27×10-4 S/cm to 1.58×10-3 S/cm.
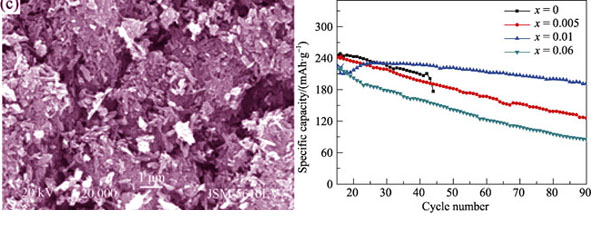
|
|
|
Si Nanowire Anode Prepared by Chemical Etching for High Energy Density Lithium-ion Battery
LI Jian-Wen, ZHOU Ai-Jun, LIU Xing-Quan, LI Jing-Ze
2013 Vol. 28 (11): 1207–1212
 Abstract
Abstract(
969 )
 HTML
HTML
 PDF
PDF(945KB)(
30300
)
Silicon nanowire arrays were fabricated by metal-assisted chemical etching of single crystal silicon wafer. Then the silicon nanowire powder was obtained via ultrasonicating from silicon wafer and utilized as the anode electrode material of lithium ion battery. The effects of Ag catalyst deposition process and the subsequent anisotropic chemical etching on the formation of silicon nanowires were systematically investigated. The redistribution of Ag particles was found during the chemical etching. The large-area silicon nanowire arrays with proper length-diameter ratio were obtained by optimizing the concentration of AgNO3, HF and H2O2 solution. The silicon nanowires exhibited the classical alloy-reaction plateaus of silicon and the specific capacity above 1800 mAh/g prior to the sixth cycle.
|
|
|
Hollow Nanotubular SnO2 Templated by Cellulose Fibers for Lithium Ion Batteries
MAO Rui, GUO Hong, TIAN Dong-Xue, YANG Xiang-Jun, WANG Shi-Xiong, CHEN Jing
2013 Vol. 28 (11): 1213–1216
 Abstract
Abstract(
849 )
 HTML
HTML
 PDF
PDF(614KB)(
1588
)
An effective method of using cellulosic substances (filter paper) as template was employed to prepare SnO2 nanotubular materials examined as anode for Li-ion battery. According to XRD, SEM, TEM and HR-TEM analysis, the synthesized nanotubular materials retained morphological hierarchy of the filter paper, and each nanotube was composed of nano-sized SnO2 ranged from 5 nm to 15 nm. At the same time, the N2 adsorption/desorption tests show that the materials are mesoporous structure. It exhibits a stable reversible capacity of 580 mAh/g at constant current density of 100 mA/g, and the reversible capacity remains at 550 mAh/g after 60 cycles, showing a high reversible capacity and stable cycle performance as anode for Li-ion battery.
|
|
|
Potentiostatic Electrodeposition of Pt-Fe Alloy Catalyst and Application in PEMFC Cathode
ZHAO Wen-Wen, ZHANG Hua, LI Mei
2013 Vol. 28 (11): 1217–1222
 Abstract
Abstract(
802 )
 HTML
HTML
 PDF
PDF(541KB)(
1436
)
Pt-Fe alloy for proton exchange membrane fuel cell (PEMFC) cathode catalyst was prepared on the porous carbon cloth by electrodeposition under the constant voltage. The reduction potential was determined by cyclic voltammetry (CV). The physical and electrochemical performances of catalysts were characterized by X-ray difffraction (XRD), scanning electron microscope (SEM), field emission scanning electron microscope (FESEM), energy dispersive spectrograph (EDS), cyclic voltammetry (CV), single cell polarization test and in-situ electrochemical impedance spectroscopy (EIS) technique. The results show that the highly-dispersed Pt-Fe alloy catalyst is obtained by electrodeposition at 0.075 V. The electrodeposition time has remarkable effect on the compositions of Pt-Fe alloy. The ratio of Pt to Fe increases with the co-deposition time. Fe can sufficiently improve the catalytic activity of Pt for oxygen reduction reaction (ORR). Pt-Fe alloy catalyst electrodeposited for 30 min shows better catalytic activity for ORR than pure Pt catalyst.
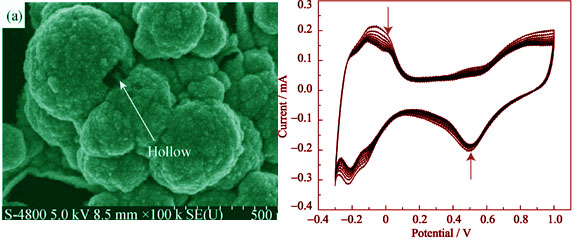
|
|
|
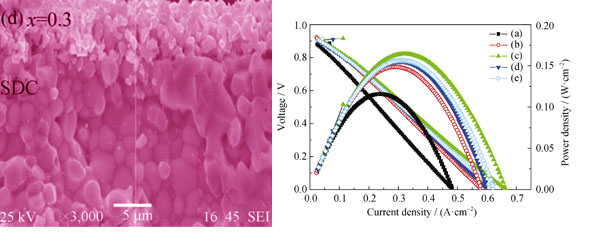
Preparation and Performance of Cathode Materials Ba1-xSrxCo0.7Fe0.2Nb0.1O3-δ for Intermediate-temperature Solid Oxide Fuel Cells
HAN Fei, LIU Xiao-Mei, BI Hai-Lin, ZHANG Li-Jun, PEI Li, SU Wen-Hui
2013 Vol. 28 (11): 1223–1227
 Abstract
Abstract(
757 )
 HTML
HTML
 PDF
PDF(511KB)(
1381
)
The Ba1-xSrxCo0.7Fe0.2Nb0.1O3-δ(x=0, 0.1, 0.2, 0.3, 0.4) oxides were synthesized by the conventional solid state reaction process and investigated as a cathode for intermediate temperature solid oxide fuel cells (IT-SOFCs). All prepared Ba1-xSrxCo0.7Fe0.2Nb0.1O3-δ(x=0-0.4) powders were sintered at 1000 ℃. X-ray diffraction patterns indicate that all samples have a single phase of cubic perovskite structure. The morphologies of the symmetric cell were characterized by a scanning electron microscope (SEM). The cathode exhibits fine uniform microstructure with appropriate porosity, and shows good bonding and continuous contact with the dense Ce0.85Sm0.15O1.925(SDC) electrolyte pellet. AC impedance spectra based on SDC electrolyte measured at intermediate temperatures show that a cathode Ba0.8Sr0.2Co0.7Fe0.2Nb0.1O3-δ exhibits the best electrochemical performance. The maximum power density of single cell with Ba0.8Sr0.2Co0.7Fe0.2Nb0.1O3-δ cathode, Ni0.9Cu0.1-SDC anode and SDC electrolyte is 155 mW/cm2 at 600℃. The encouraging results suggest that Ba0.8Sr0.2Co0.7Fe0.2Nb0.1O3-δ is a very promising cathode material for IT-SOFCs.
|
|
|
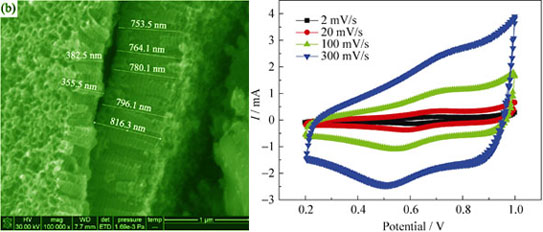
Preparation and Electrochemical Properties of Ti/TiO2-MnO Nano Array Electrode for Supercapacitor
HUANG You-Guo, ZHENG Feng-Hua, REN Meng-De, LI Qing-Yu, WANG Hong-Qiang
2013 Vol. 28 (11): 1228–1232
 Abstract
Abstract(
850 )
 HTML
HTML
 PDF
PDF(435KB)(
1751
)
A Ti/TiO2-MnO composite electrode was prepared by anodizing, chemical dipping and annealing. The crystalline structure and the microscopic morphology of the sample were characterized by XRD and SEM. The results show that the pore diameters of the Ti/TiO2 nanotube arrays (NTAs) range from 60 nm to 95 nm, and their lengths range from 350 nm to 820 nm. Ti/TiO2 nanotube array obtained through anodizing at ambient temperature is amorphous. However, it transforms from amorphous to anatase after annealing at 500℃. Only MnO is found after the sample is immersed in MnSO4 solution. A button supercapacitor device is assembled and its cyclic voltammetry curves are determined. It shows that its cyclic voltammetry curves have a pair of redox peaks, which corresponds to the calation/intercalation process of H+ in NTAs. The intercalation/calation process of H+ is controlled by diffusion. The CV response current of Ti/TiO2-MnO electrode decreases as TiO2 transforms from amorphous structure to anatase and MnO deposites in NTAs.
|
|
|
Preparation and Electrical Properties of TiO2/SnO2 Nanocrystalline Films
CUI Xu-Mei, ZUO Cheng-Yang, LAN De-Jun, WANG Jun
2013 Vol. 28 (11): 1233–1236
 Abstract
Abstract(
748 )
 HTML
HTML
 PDF
PDF(356KB)(
1433
)
The effect of SnO2 nanocrystalline coatings on the performance of dye-sensitized solar cells (DSSC) based on TiO2-nanoparticle photoanode was studied. The TiO2-nanoparticle photoanode was prepared by screen-printing technique, and the SnO2 nanocrystalline films were coated on TiO2 nanoparticles by soaking TiO2 photoanodes in SnO2 solution with different concentrations or different time. SEM images indicate SnO2 nanocrystalline films have smaller surface grains than the TiO2 nanocrystalline films. The electrical properties of the films indicate that SnO2 thin films growing on the TiO2 films by soaking TiO2 films in 0.4 mol /L SnO2 solution for 50 min play a positive role on the structure and performance of the TiO2 films, and the conversion efficiency of the solar cell with TiO2/SnO2 photoanode is about 7% higher than that of TiO2 films.
|
|
|
Theoretical Studies on the Small Sized Lithium-silicon Clusters SinLi (n=1-10)
WANG Zhi-Qiang, LEI Xue-Ling, WU Mu-Sheng, LIU Gang, XU Bo, OUYANG Chu-Ying
2013 Vol. 28 (11): 1237–1242
 Abstract
Abstract(
570 )
 HTML
HTML
 PDF
PDF(401KB)(
1424
)
Theoretical studies on the structures, stabilities and electronic properties of SinLi (n=1-10) clusters were carried out by the density functional theory (DFT). The results show that lithium atom prefers to stay at the surface of cluster and likes to be adsorbed on the bridge site. The binding energies indicate that the stabilities of SinLi clusters can be enhanced by adsorption of lithium atom. Additionally, lithium atom binding energies show that the interaction between lithium atom and silicon cluster increases as decreasing the cluster size, which is an important factor for the amorphization process upon lithiation of the silicon based anode materials for Li-ion batteries. The ionization potential, electron affinity potential, chemical potential and energy gap of the SinLi clusters all indicate that Si4Li and Si7Li are easier to lose α electron to form cationic clusters.
|
|
|
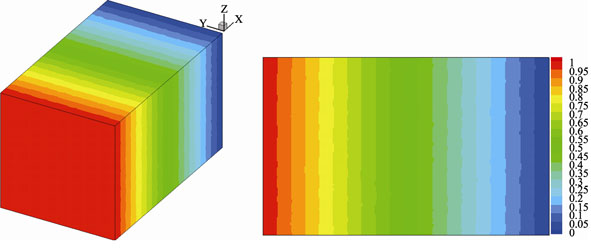
3D Monte Carlo Reconstruction and Characterization of LiCoO2 Cathode
WU Wei, JIANG Fang-Ming, CHEN Zhi, WANG Ying, ZHAO Feng-Gang, ZENG Yu-Qun
2013 Vol. 28 (11): 1243–1247
 Abstract
Abstract(
782 )
 HTML
HTML
 PDF
PDF(516KB)(
1490
)
Reconstruction and characterization of the porous composite electrode via experimental and numerical approaches are the basis and prerequisite of pore-scale modeling. The Monte Carlo approach was employed to reconstruct the LiCoO2 cathode of a Li-ion battery. The reconstructed electrode resolves sub-micrometer microstructure, thus evidently distinguishing the three individual phases: LiCoO2 as active material, pores (electrolyte), and additives. An extensive characterization was subsequently performed to calculate some important structural parameters and transport properties, including the geometrical connectivity and the specific surface area, etc. Particularly, a self-developed D3Q15 LB (Lattice Boltzmann) model was used to calculate the effective thermal (or electric) conductivity and the effective species diffusivity in electrolyte (or solid) phase, and the tortuosity of an individual phase. The reconstructed 3D microstructure is consistent with the real cathode microstructure concerning several important statistical features including porosity, volume fraction of each phase, and two-point correlation functions etc. LB model predictions indicate that the effective transport coefficients are closely related to the micro-morphology in electrodes.
|
|
|
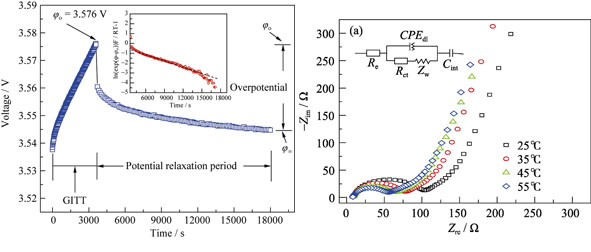
Preparation and Electrochemical Properties of LiMn0.8Fe0.2PO4/C Nanocomposite
SU Jing, WU Xing-Long, GUO Yu-Guo
2013 Vol. 28 (11): 1248–1254
 Abstract
Abstract(
786 )
 HTML
HTML
 PDF
PDF(644KB)(
1441
)
Olivine-structured LiMn0.8Fe0.2PO4/C solid solution was successfully prepared by a Sol-Gel approach combined with a high-temperature solid-state reaction, and characterized by X-ray diffraction (XRD), scanning electron microscope (SEM) and transmission electron microscope (TEM). The results revealed that LiMn0.8Fe0.2PO4 nanoparticles were evenly dispersed in the in-situ formed carbon conductive network. When the nanocomposite served as a cathode material for lithium-ion batteries (LIBs), except a short plateau (~3.5 V vs Li+/Li) ascribed to Fe3+/Fe2+ couples in the charging/discharging profile, the long plateau at higher voltage (~4.1 V vs Li+/Li) is corresponding to the redox reaction of Mn during the Li+ extraction/insertion in the LiMn0.8Fe0.2PO4 lattice, and this high voltage plateau can significantly enhance the energy density of relevant LIBs. In addition, the chemical diffusion behavior of lithium in the LiMn0.8Fe0.2PO4/C electrode was investigated in detail using galvanostatic intermittent titration technique (GITT) and electrochemical impedance spectroscope (EIS), and the chemical diffusion coefficients of lithium, DLi, obtained by GITT and EIS were 5×10-15 -1×10-14 cm2/s and 1.27×10-13- 2.11×10-13 cm2/s, respectively. The results indicate that the value of DLi increases with elevated test temperature, and it can be inferred that the electrochemical properties of such materials can be improved by raising the working temperature.
|
|
|
Sol-Gel Synthesis and Conductivity Properties of Sodium Ion Solid State Electrolytes Na3Zr2Si2PO12
ZHANG Zhi-Zhen, SHI Si-Qi, HU Yong-Sheng, CHEN Li-Quan
2013 Vol. 28 (11): 1255–1260
 Abstract
Abstract(
1396 )
 HTML
HTML
 PDF
PDF(473KB)(
1813
)
NASICON-structured Na3Zr2Si2PO12 was synthesized by a Sol-Gel approach. Phase-pure samples were successfully sintered at 1050℃ when adding 10% excessive Na and P in the precursors, while a small amount of ZrO2 impurity was detected without adding excessive phosphorus. Electrochemical impedance spectrum tests indicate that the ionic conductivity of the former is as high as 5.4×10-4 S/cm at room temperature, which is higher than that of samples prepared from the precursors without adding excessive phosphorus (3.7×10-4 S/cm). Further analysis reveals that the evaporation of phosphorus at high temperature would cause the formation of ZrO2 impurity in the samples, leading to a lower ionic conductivity. Compared with solid state reaction approach, samples with enhanced ionic conductivity can be obtained at a rather lower temperature by Sol-Gel synthesis.
|
|
|
Improved Electrochemical Properties of Al3+-doped 0.5Li2MnO3-0.5LiCo1/3Ni1/3Mn1/3O2 Cathode for Lithium Ion Batteries
ZHANG Wen-Hua, HE Wei, PEI Feng, WU Fa-Yuan, MAO Rong-Jun, AI Xin-Ping, YANG Han-Xi, CAO Yu-Liang
2013 Vol. 28 (11): 1261–1264
 Abstract
Abstract(
690 )
 HTML
HTML
 PDF
PDF(378KB)(
1488
)
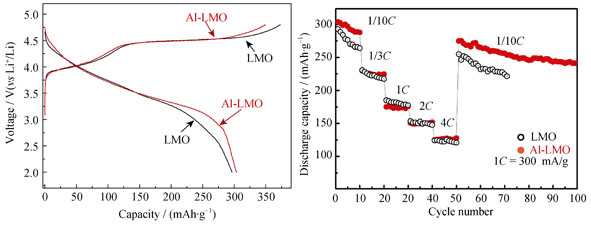
The lithium-rich cathode materials Li[Li0.2Co0.13Ni0.13Mn0.51Al0.03]O2 doped with 3% Al3+ were synthesized by a polymer-pyrolysis method. The structure and morphology of the as-prepared material were characterized by X-ray diffraction (XRD), scanning electron microscope (SEM). The structural analyses exhibit that the Al3+-doped sample maintains the layered structure of the Li-rich oxide material and no impurity is detected in XRD patterns. The charge-discharge tests show that the Al3+-doped sample has a high charge/discharge capacity of 349.1/303.8 mAh/g (initial coulombic efficiency of 87%) at current density of 30 mA/g. Additionally, Al3+-doped sample also exhibits excellent cyclability with 91.7% capacity retention over 100 cycles. The results demonstrate that Al3+ doping is favorable to maintain the structural stability of the layered structure during the electrochemical lithium insertion/extraction, so as to provide a promising route to develop cathode materials with high capacity and stability.
|
|
|
Structure and Electrochemical Performance of LiFePO4/C Cathode Materials Coated with Nano Al2O3 for Lithium-ion Battery
ZHAO Shi-Xi, LI Ying-Da, DING Hao, LI Bao-Hua, NAN Ce-Wen
2013 Vol. 28 (11): 1265–1269
 Abstract
Abstract(
845 )
 HTML
HTML
 PDF
PDF(374KB)(
1425
)
The structure and electrochemical performance of Al2O3-coated LiFePO4 cathode materials were investigated. A nano Al2O3 coating on the surface of commercial LiFePO4/C particles (Aleees Inc.) was prepared by using the Sol-Gel method. The effect of the amount of Al2O3 coating on the electrochemical performance and structural stability of LiFePO4 electrodes at room temperature (25℃) and elevated temperatures (55℃) was studied. The results show that 2wt% Al2O3 coating can effectively enhance the cycling capacity, alleviate capacity fading at high temperature and reduce cell impedance. It is largely attributed to Al2O3 coating, which plays a regulatory role of lithium-ion inserting the lattice and preventing direct contact between the cathode material and the electrolyte, and improves the structural stability of LiFePO4 during cycles.
|
|
|
NiO-doped CaCu3Ti4O12 Thin Film by Sol-Gel Method
XU Dong,SONG Qi, ZHANG Ke, XU Hong-Xing, YANG Yong-Tao, YU Ren-Hong
2013 Vol. 28 (11): 1270–1274
 Abstract
Abstract(
1483 )
 HTML
HTML
 PDF
PDF(389KB)(
1769
)
Pure CaCu3Ti4O12 (CCTO) thin film and NiO-doped CaCu3-xNixTi4O12 (CCNTO) thin film with x=0.10, 0.20 and 0.30 were prepared by Sol-Gel method. The effects of NiO on the dielectric properties and microstructure revolution of CCTO were studied. The crystalline structure and the surface morphology of the films were markedly influenced by NiO content. AFM images of the CaCu3-xNixTi4O12 samples showed that the grain size of Ni-doped CCTO was smaller than the grain size of CCTO without Ni doping. For the CCNTO thin film(x=0.2), the lowest leakage current was obtained and the minimum value reached 0.564 mA. Meanwhile, the highest threshold voltage and nonlinear coefficient were 81 V/mm and 1.9, respectively. The dielectric constant increased when the doping content of Ni reached a certain level. Besides, the dielectric loss was decreased firstly, and then increased.
|
|