全无机钙钛矿量子点CsPbX3 (X=Cl, Br, I)具有吸收系数高、发光效率高、发光在整个可见光范围内可调和发射谱线窄等优异性能, 引起了广泛关注。作为新型发光材料, 钙钛矿量子点在发光二极管、太阳能电池、光探测和激光等领域具有广阔的应用前景, 是近年来光电材料的研究热点[1,2,3,4]。然而, 它的离子结构特殊, 造成其稳定性很差, 从而限制了实际应用。其中, 水和氧气是影响钙钛矿量子点环境稳定性的关键因素。当这类材料暴露在空气中时, 与水和氧气的接触会使其发生快速分解而破坏离子结构。 因此, 稳定性问题限制了该材料在水相中的应用, 如光催化、光电催化以及生物检测等[5,6,7]。因此, 开发具有水稳定性的钙钛矿量子点, 对于进一步拓展全无机钙钛矿量子点在水环境中的应用具有十分重要的意义。
近年来, 研究者采取了大量措施来解决CsPbX3钙钛矿量子点的稳定性问题, 包括引入防水配位基进行表面钝化、掺杂其他离子以及采用无机或者有机保护层进行包覆等[8,9,10,11,12]。表面钝化和离子掺杂虽然可以在一定程度上提高CsPbX3钙钛矿量子点的稳定性, 但是这两种方法都有其局限性, 例如, 配位基在CsPbX3表面和溶液之间存在动态配体交换过程, 在高湿度、极性溶剂或者高温条件下会严重破坏CsPbX3的结构; 而离子掺杂并没有改变CsPbX3的初始晶体结构, 致使其稳定性提高有限。与前两种方法不同, 包覆是在CsPbX3的表面引入具有较高机械强度和不透气的有机或者无机化合物保护层。这些保护层通过包覆CsPbX3钙钛矿量子点, 有效防止CsPbX3与水、氧气等接触。并且包覆还可以防止CsPbX3钙钛矿量子点在溶液中的团聚, 使其保持稳定性。因此, 包覆是提高CsPbX3钙钛矿量子点稳定性最有效和最直接的方法。
Li等[19]采用TiO2对CsPbBr3钙钛矿量子点进行包覆, 大幅提升了CsPbBr3在水中的稳定性, 并利用TiO2壳层的导电性提升了CsPbBr3/TiO2复合材料的电荷分离效率。在此基础上, 为了拓展CsPbBr3钙钛矿量子点在液相光催化领域的应用, 本工作制备了CsPbBr3@TiO2核壳结构纳米复合材料, 并研究了该材料降解水中有机污染物罗丹明B的光催化性能和稳定性。
1 实验方法
1.1 实验试剂
碳酸铯(Cs2CO3, 99.9%), 油酸(C18H34O2, >90%); 十八烯(C18H36, >90%), 油胺(C8H19N, >90%), 溴化铅(PbBr2, 99.999%), 丙酮(CH3COCH3, 99.5%), 甲苯(C7H8, 99.5%), 钛酸四丁酯(C16H36O4Ti, 99%)。以上化学试剂均购于上海泰坦集团。
1.2 材料的制备
1.2.1 CsPbBr3钙钛矿量子点的制备
参考文献[20]的制备方法, 采用热注入法合成CsPbBr3量子点。在氩气环境中, 将0.8 g Cs2CO3, 2.5 mL油酸和30 mL十八烯的混合物放入100 mL的四颈烧瓶中脱气, 在130 ℃下保温1 h。然后将反应温度升至150 ℃再保温0.5 h, 直到所有的Cs2CO3与油酸反应, 经自然冷却至室温后, 得到Cs前驱体。将十八烯(10 mL), 油酸(1 mL)、油胺(1 mL)和溴化铅(0.36 mmol)混合, 在130 ℃氩气气氛中脱气1 h。等到溴化铅完全溶解之后, 将温度升至160 ℃再保温10 min。然后将1 mL Cs前驱体迅速注入上述热混合物中, 并在5 s后用冰浴停止反应, 得到CsPbBr3钙钛矿量子点。通过添加过量丙酮使CsPbBr3纳米晶沉淀, 并进行离心分离, 用甲苯和丙酮的混合溶液洗涤产物。最后, 将产物分散在甲苯中以供进一步使用。
1.2.2 CsPbBr3@TiO2复合材料的制备
参考文献[19]方法制备CsPbBr3@TiO2复合材料。将20 μL钛酸四丁酯溶解在1 mL甲苯中, 在磁力搅拌下, 再将其滴加至10 mL浓度为 1 mg/mL的CsPbBr3量子点的甲苯溶液中。在温度为25 ℃, 湿度为30%的手套袋中搅拌3 h, 离心分离得到产物, 并在80 ℃下真空干燥12 h。将所得的粉末放入管式炉中, 在氩气保护气氛下升温至300 ℃并煅烧5 h。自然冷却至室温, 得到TiO2包覆CsPbBr3钙钛矿量子点材料。
1.3 材料的表征
采用X 射线衍射仪(XRD, D/max 2200PC, 铜靶电压40 kV, 电流40 mA)测试样品的物相。采用透射电子显微镜(TEM, FEI tecnaiG2F30, 200 kV)表征样品的显微结构。采用X射线光电子能谱仪(XPS, ESCALAB 250Xi)对样品进行成分标定及元素化学状态分析。采用傅里叶变换红外光谱仪(FT-IR, Nicolet iN10)研究材料的分子结构和化学键。采用电化学工作站(CHI 650E, 上海辰华)测试样品的光电流。采用三电极系统测试光电流, 以涂敷样品的FTO玻璃做工作电极, 饱和甘汞电极做参比电极, 铂丝做对电极。电解质为0.1 mol/L的Na2SO4溶液, 光源为500 W的氙灯。
1.4 材料的光催化性能表征
称量0.05 g的催化剂, 将其超声分散于50 mL罗丹明B 溶液(10-5 mol/L)中, 得到的悬浮液在避光条件下搅拌0.5 h以达到吸附平衡, 然后将其置于500 W氙灯(采用420 nm滤波片滤去紫外光)下进行可见光照射。每隔10 min取样一次, 对所取溶液离心分离后, 用紫外-可见分光光度计(PE Lambda 900)测试罗丹明B 溶液在最大吸收波长552 nm 处的吸光度。
2 结果与讨论
2.1 XRD分析
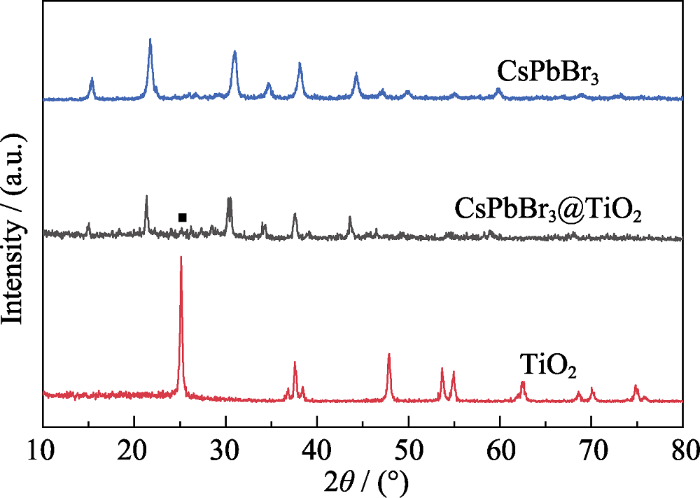
图1为CsPbBr3钙钛矿量子点、TiO2和CsPbBr3@TiO2复合材料的XRD图谱。由图可知, CsPbBr3钙钛矿量子点在2θ=15.2°, 21.5°, 26.3°, 30.7°, 34.5°, 37.6°和43.7°处均出现了明显的特征峰, 分别对应CsPbBr3量子点的(100), (110), (111), (200), (210), (211)和(202)晶面。所有的XRD特征峰对应单斜相CsPbBr3的标准卡(JCPDS 18-0364), 说明制备的CsPbBr3量子点具有单斜相结构。相比于纯CsPbBr3钙钛矿量子点, CsPbBr3@TiO2复合材料的XRD图谱没有明显的变化, 只是在2θ=25.1°处出现了锐钛矿相TiO2的最强衍射峰, 说明在包覆过程中CsPbBr3钙钛矿量子点的相结构没有遭到破坏。另一方面, TiO2只检测到2θ=25.1°处的最强衍射峰, 可能是由于TiO2结晶不完全, 含有非晶态的TiO2。
图1
图1
CsPbBr3、TiO2和CsPbBr3@TiO2的XRD图谱
Fig. 1
XRD patterns of CsPbBr3, TiO2 and CsPbBr3@TiO2
2.2 TEM分析
图2
图2
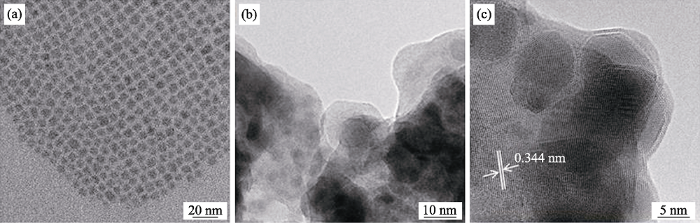
CsPbBr3(a)和CsPbBr3@TiO2(b)的TEM照片; (c)CsPbBr3@TiO2的高分辨TEM照片
Fig. 2
TEM images of CsPbBr3 (a) and CsPbBr3@TiO2 (b), and (c) HRTEM image of CsPbBr3@TiO2
2.3 傅里叶变换红外光谱分析
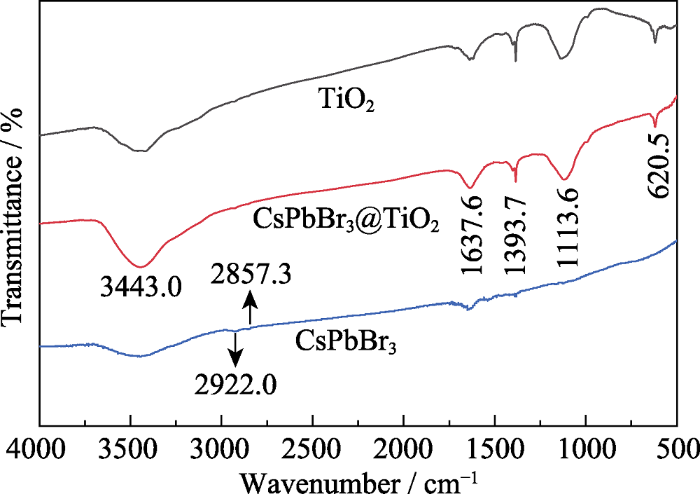
图3是CsPbBr3钙钛矿量子点、TiO2和CsPbBr3@TiO2复合材料的傅里叶变换红外光谱图, 可以看出, 样品在3443.0 cm-1处都表现出明显的吸收信号, 对应于表面羟基的伸缩振动模式。CsPbBr3钙钛矿量子点在2922.0和2857.3 cm-1处有两个吸收峰, 归属于-CH3的反对称拉伸模式。CsPbBr3@TiO2 复合材料的红外光谱中, 1637.6和1393.7 cm-1处的吸收峰来自于表面羟基的伸缩振动, 1113.6和620.5 cm-1处的吸收峰则来自于Ti-O键的不对称振动模式[21,22]。另外, 可以看出CsPbBr3@TiO2复合材料和纯TiO2的红外光谱基本一致, 说明TiO2包覆在CsPbBr3钙钛矿量子点的外面, 导致无法探测到内层CsPbBr3钙钛矿量子点的吸收峰。
图3
图3
CsPbBr3、TiO2和CsPbBr3@TiO2的傅里叶变换红外光谱
Fig. 3
FT-IR spectra of CsPbBr3, TiO2 and CsPbBr3@TiO2
2.4 XPS分析
图4
图4
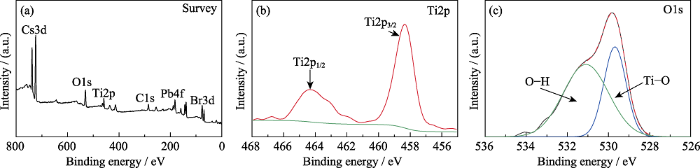
(a)CsPbBr3@TiO2的XPS全谱图; (b)Ti2p和(c)O1s的XPS 高分辨分峰拟合谱
Fig. 4
Survey XPS spectrum of CsPbBr3@TiO2 (a), and high resolution XPS spectra of Ti2p (b) and O1s (c)
2.5 光催化性能表征
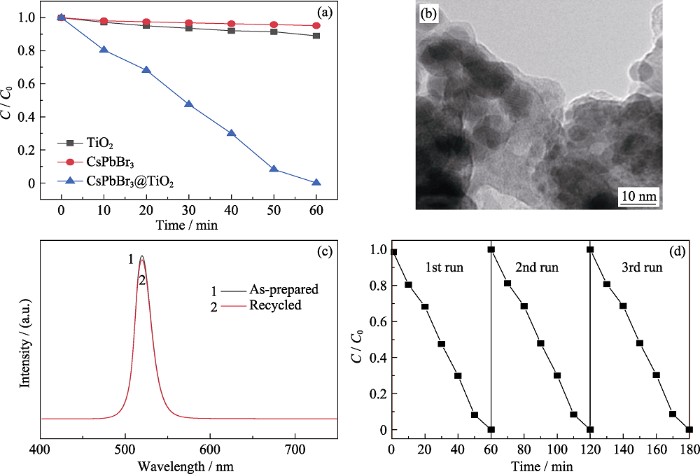
通过在可见光下降解模拟污染物罗丹明B表征CsPbBr3@TiO2复合材料的光催化性能, 结果如图5(a)所示, 可以看出, CsPbBr3@TiO2复合材料作为光催化剂的条件下, 随着光照时间的延长, 罗丹明B逐渐被降解, 光照60 min时降解完全。而在单CsPbBr3或TiO2的作用下, 罗丹明B几乎没有降解。这是因为TiO2在可见光下没有响应, 导致其可见光催化性能较差。而CsPbBr3钙钛矿量子点由于在水中结构不稳定, 导致其在水中很快发生分解而失去光催化活性。而在包覆TiO2之后, TiO2可以作为保护层将CsPbBr3和水分隔开来, 从而防止CsPbBr3分解。从图5(b)可以看出, 经过光催化降解实验之后, 回收的CsPbBr3@TiO2复合材料保持了原有的核壳结构, 说明包覆TiO2确实能够保持CsPbBr3钙钛矿量子点在水中的结构稳定性。为了进一步证实CsPbBr3@TiO2复合材料在水中的稳定性, 对光催化降解实验前后材料的PL谱进行了测试, 结果如图5(c)所示, CsPbBr3@TiO2复合材料的PL谱在光催化降解实验前后没有明显的变化。除此之外, 循环实验表明, 经过三次循环测试, CsPbBr3@TiO2复合材料降解罗丹明B的光催化性能没有明显的下降(图5(d)), 证明该材料具有较高的水稳定性。
图5
图5
(a) CsPbBr3、TiO2和CsPbBr3@TiO2 在可见光下降解罗丹明B的光催化性能比较; (b)降解实验后回收的CsPbBr3@TiO2复合材料的TEM照片; (c)降解实验前后CsPbBr3@TiO2的PL光谱对比图; (d) CsPbBr3@TiO2降解罗丹明B的循环实验
Fig. 5
(a) Comparison of photocatalytic activities of CsPbBr3, TiO2 and CsPbBr3@TiO2 for degradation of Rhodamine B under visible light irradiation; (b) TEM image of recycled CsPbBr3@TiO2 after the degradation experiment; (c) Comparison of PL spectra of CsPbBr3@TiO2 before and after the degradation experiment; (d) Cycle runs of the photocatalytic degradation of RhB over CsPbBr3@TiO2
2.6 光催化反应机理
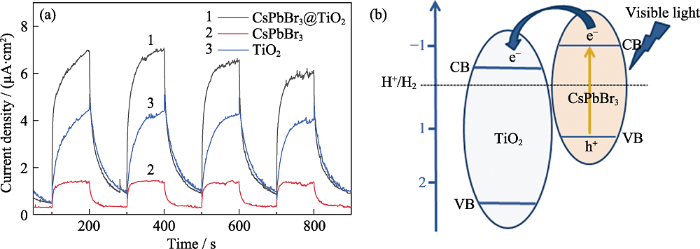
光电流可以直接反映光催化剂的载流子分离效率, 而载流子的分离效率和材料的光催化性能密切相关[24]。为了研究CsPbBr3@TiO2复合材料的光催化性能提升机理, 测试了样品的光电流, 结果如图6(a)所示。可以看出, 包覆TiO2之后, CsPbBr3钙钛矿量子点的光电流有了大幅提升, 分别为纯CsPbBr3钙钛矿量子点和TiO2的6倍和1.5倍左右, 说明CsPbBr3钙钛矿量子点与TiO2复合能够有效提升材料的载流子分离效率。在实际光催化的过程中, 载流子的分离效率是影响光催化性能的关键因素, 它直接影响光催化剂的最终效率, 而构建异质结可以有效提高载流子的分离效率。在各种形式的异质结中, II型异质结和直接型Z-scheme是两种最典型的提高载流子分离效率的方式[25,26]。II型异质结和直接型Z-scheme都是由两种半导体材料组成, 其中II型异质结由于两种半导体材料的能级位错可以促进光生载流子在界面间的电荷转移, 从而有利于载流子的分离。根据文献可知, CsPbBr3钙钛矿量子点的价带和导带位置分别为1.5和-1.0 eV[27], 而TiO2的价带和导带位置分别为2.7和-0.5 eV [28]。因此, CsPbBr3钙钛矿量子点的导带位置高于TiO2, 而其价带位置也在TiO2的价带位置之上。两者的能级交错形成II型异质结。在可见光的激发下, 钙钛矿量子点产生的光生电子可以转移到TiO2的导带上, 抑制CsPbBr3钙钛矿量子点中光生载流子的复合, 促进光生载流子的分离(如图6(b)所示), 从而提升光催化性能。
图6
图6
(a)CsPbBr3、TiO2和CsPbBr3@TiO2的光电流; (b)CsPbBr3@TiO2复合材料的光催化机理示意图
Fig. 6
(a) Photocurrent responses of CsPbBr3, TiO2 and CsPbBr3@TiO2, and (b) Photocatalytic mechanism of CsPbBr3@TiO2 composite
3 结论
利用钛酸四丁酯的水解并煅烧, 成功地在CsPbBr3钙钛矿量子点表面包覆TiO2壳层, 制备了CsPbBr3@TiO2核壳结构纳米复合材料。TiO2作为保护层, 大幅提升了CsPbBr3钙钛矿量子点在水中的结构稳定性。经过光催化降解罗丹明B之后, CsPbBr3@TiO2复合材料的形貌、发光和光催化性能没有发生明显的变化。并且, TiO2包覆还提高了CsPbBr3@TiO2复合材料的载流子分离效率, 从而提升了材料的光催化性能。本研究为使用卤化物钙钛矿量子点作为可见光催化剂提供了新的思路。
参考文献
Interface engineering of highly efficient perovskite solar cells
Advancing perovskite solar cell technologies toward their theoretical power conversion efficiency (PCE) requires delicate control over the carrier dynamics throughout the entire device. By controlling the formation of the perovskite layer and careful choices of other materials, we suppressed carrier recombination in the absorber, facilitated carrier injection into the carrier transport layers, and maintained good carrier extraction at the electrodes. When measured via reverse bias scan, cell PCE is typically boosted to 16.6% on average, with the highest efficiency of ~19.3% in a planar geometry without antireflective coating. The fabrication of our perovskite solar cells was conducted in air and from solution at low temperatures, which should simplify manufacturing of large-area perovskite devices that are inexpensive and perform at high levels.
Improving the stability of metal halide perovskite quantum dots by encapsulation
CsPbX3 quantum dots for lighting and displays: room-temperature synthesis, photoluminescence superiorities, underlying origins and white light-emitting diodes
Emissions at perovskite quantum dot/film interface with halide anion exchange
Photocatalytic gydrogen generation from hydriodic acid using methylammonium lead iodide in dynamic equilibrium with aqueous solution
Metal halide perovskite nanomaterials: synthesis and applications
All inorganic halide perovskites nanosystem: synthesis, structural features, optical properties and optoelectronic applications
Genesis challenges and opportunities for colloidal lead halide perovskite nanocrystals
Colloidal metal halide perovskite nanocrystals: synthesis characterization and applications
Role of the chemical substitution on the structural and luminescence properties of the mixed halide perovskite thin MAPbI3-xBrx(0≤x≤1) films
Colloidal nanocrystals embedded in macrocrystals: robustness, photostability, and color purity
Solvent-assisted self-assembly of gold nanorods into hierarchically organized plasmonic mesostructures
Plasmonic supercrystals and periodically structured arrays comprise a class of materials with unique optical properties that result from the interplay of plasmon resonances, as well as near- and far-field coupling. Controlled synthesis of such hierarchical structures remains a fundamental challenge, as it demands strict control over the assembly morphology, array size, lateral spacing, and macroscale homogeneity. Current fabrication approaches involve complicated multistep procedures lacking scalability and reproducibility, which has hindered the practical application of plasmonic supercrystal arrays. Herein, these challenges are addressed by adding an organic solvent to achieve kinetic control over the template-assisted colloidal assembly of nanoparticles from aqueous dispersion. This method yields highly regular periodic arrays, with feature sizes ranging from less than 200 nm up to tens of microns. A combined experimental/computational approach reveals that the underlying mechanism is a combination of the removal of interfacial surfactant micelles from the particle interface and altered capillary flows. Assessing the efficacy of such square arrays for surface-enhanced Raman scattering spectroscopy, we find that a decrease of the lattice periodicity from 750 nm down to 400 nm boosts the signal by more than an order of magnitude, thereby enabling sensitive detection of analytes, such as the bacterial quorum sensing molecule pyocyanin, even in complex biological media.
Highly efficient and stable blue-emitting CsPbBr3@SiO2 nanospheres through low temperature synthesis for nanoprinting and wled
CsPbBr3 QD/AlOx inorganic nanocomposites with exceptional stability in water light and heat
CsPbBr3 nanocrystal/MO2(M=Si, Ti, Sn) composites: insight into charge-carrier dynamics and photoelectrochemical applications
CsPbX3 nanocrystals films coated on YAG: Ce3+ pig for warm white lighting source
Highly stable and luminescent perovskite-polymer composites from a convenient and universal strategy
In situ fabrication of halide perovskite nanocrystal-embedded polymer composite films with enhanced photoluminescence for display backlights
Photoelectrochemically active and environmentally stable CsPbBr3/TiO2 core/shell nanocrystals
Nanocrystals of cesium lead halide perovskites (CsPbX3, X = Cl, Br, and I): novel optoelectronic materials showing bright emission with wide color gamut
Construction of highly ordered ZnO-TiO2 nanotube arrays (ZnO/TNTs) heterostructure for photocatalytic application
In recent years, strenuous efforts have been devoted to exploring ZnO functionalized TiO(2) nanotube arrays (ZnO/TNTs) nanocomposites; however, there is still a paucity of reports on the construction of well-defined ZnO/TNTs heterostructure via efficient and easily accessible approach. In this work, drawing on a two-step anodization combined pyrolysis strategy, we attained a highly ordered ZnO/TNTs hybrid nanostructure. Combined with a collection of characterizations including X-ray photoelectron spectroscopy (XPS), X-ray diffraction (XRD), diffusion reflectance spectrum (DRS), scanning electron microscopy (SEM), and transmission electron microscopy (TEM), we found that, in this coupling, in situ formed ZnO phases were uniformly grafted to TNTs framework giving rise to hybrid nanostructure, which is ascribed to cooperative interfacial interaction between polar TiO(2) layer and ZnO precursor. The underlying interaction leading to judicious combination of TNTs and ZnO was unveiled by Fourier transformed infrared spectrum (FTIR) and XPS. Alternatively, it has been shown that ZnO nanocrystals distributed on the TNTs could serve as favorable hole channels and receptors for efficient separation of photoexcited charge carriers, which results in significantly enhanced photocatalytic performances of ZnO/TNTs heterostructure in comparison with pure TNTs, ZnO film, and P25 particulate film. Furthermore, it is found that the hybrid photocatalyst demonstrated excellent photostability. It is hoped that our work could present a straightforward paradigm for preparation of hierarchical semiconductor/1-D semiconductor heterostructures.
Sonochemical synthesis of nanocrystalline TiO2 by hydrolysis of titanium alkoxides
Amorphous TiO2 shells: a vital elastic buffering layer on silicon nanoparticles for high-performance and safe lithium storage
Photocatalytic nanodiodes for visible light photocatalysis
In-situ construction of all-solid-state Z-scheme g-C3N4/TiO2 nanotube arrays photocatalyst with enhanced visible-light-induced properties
Oxidative polyoxometalates modified graphitic carbon nitride for visible-light CO2 reduction
Precise control of quantum confinement in cesium lead halide perovskite quantum dots via thermodynamic equilibrium
Cesium lead halide (CsPbX3) nanocrystals have emerged as a new family of materials that can outperform the existing semiconductor nanocrystals due to their superb optical and charge-transport properties. However, the lack of a robust method for producing quantum dots with controlled size and high ensemble uniformity has been one of the major obstacles in exploring the useful properties of excitons in zero-dimensional nanostructures of CsPbX3. Here, we report a new synthesis approach that enables the precise control of the size based on the equilibrium rather than kinetics, producing CsPbX3 quantum dots nearly free of heterogeneous broadening in their exciton luminescence. The high level of size control and ensemble uniformity achieved here will open the door to harnessing the benefits of excitons in CsPbX3 quantum dots for photonic and energy-harvesting applications.
Thin amorphous TiO2 shell on CdSe nanocrystal quantum dots enhances photocatalysis of hydrogen evolution from water