随着印染行业的快速发展, 由此产生的大量染料废水造成了严重的环境污染, 开发高效绿色的染料废水降解技术日益迫切[1,2]。目前, 光催化降解技术[3,4]是一项应用前景广阔的废水处理技术, 主要利用半导体材料受光激发产生电子-空穴对与材料表面的O2或者•OH反应生成具有强氧化性的活性基团来将有机染料分子矿化为CO2和H2O等物质。但是, 光催化技术存在太阳光利用率低、受透光度影响、无光或弱光条件下无响应等缺点[5,6,7,8,9]。而机械催化技术[10]是最近发展起来的, 具有环保、降解效率高、无毒性等特点的一种新型染料废水处理技术。通常情况下, 机械催化技术是利用铁电材料的压催化效应[11], 通过振动压电材料在电解质固体或溶液中诱导氧化还原反应。换而言之, 该技术是利用机械振动能诱导催化剂颗粒表面产生正负电荷, 形成氧化能力很强的活性自由基, 进而降解有机染料分子。通常采用超声空化作为机械振动源, 这些机械力作用到催化剂上足以使其表面弯曲并由于压电效应而产生一个带电表面。例如, Xu等[12]利用压电材料BaTiO3机械催化降解甲基橙染料, 降解矿化效率高; Jia等[13,14,15]分别采用Pd(Ti, Zr)O3、BiFeO3、BaTiO3等压电材料机械降解有机染料, 并且取得了突破性进展。然而, 目前关于机械催化降解染料的报道主要集中在无机半导体材料领域[12,13,14,15]。
金属有机骨架材料(Metal-organic frameworks, MOF)是由金属离子和有机配体通过自组装的方式, 以金属或金属团簇为顶点, 通过刚性或半刚性的有机配体连接而成的一类新型多孔材料, 具有超高的比表面积、孔隙率及结构易调控和修饰等特点[16,17,18,19,20,21,22,23,24], 在多相催化领域显示出良好的应用前景。自从Garcia等[25]首次报道MOF-5作为光催化剂降解苯酚以来, 已有很多MOF材料用于紫外光或可见光催化降解有机染料。例如, UiO-66系列材料具有光电半导体性质被广泛用于可见光催化降解染料[26,27,28,29]、水分解制氢[30,31,32,33]、CO2还原[34,35]、有机合成[36,37]等反应。然而, MOF材料作为类铁电材料, 在机械催化降解染料方面的应用还鲜有报道[38]。最近, Zeng等[39]报道了UiO-66类型的MOF材料具有很好的类铁电响应, 相比于未功能化的MOF材料, UiO-66具有-NH2、-OH和-COOH官能团, 显示出更大的压电/铁电性能。
基于上述分析, 本研究采用溶剂热法合成具有八面体形状的NH2-UiO-66(Zr), 表征其各项物化性质; 同时, 以罗丹明B为模拟污染物, 考察其超声振动催化降解有机染料的性能, 并对其催化机理进行初步分析。
1 实验方法
1.1 实验药品
2-氨基对苯二甲酸(H2ATA, 95%)、四氯化锆(ZrCl4, 99.5%)、甲醇(MeOH, 99.5%)均购自上海百灵威科技有限公司; N,N-二甲基甲酰胺(DMF, 98.0%)、罗丹明B(RhB, AR)、异丙醇(IPA, AR)、对苯醌(BQ, AR)、乙二胺四乙酸二钠(EDTA, AR)均购自国药试剂; 超纯水自制。
1.2 NH2-UiO-66的制备
称取0.24 g ZrCl4和0.186 g H2ATA置于100 mL反应釜中, 加入60 mL DMF和1.5 mL水, 充分溶解后将反应釜转移至烘箱, 120 ℃晶化24 h。产物用DMF离心洗涤除去未反应的配体, 之后用MeOH洗去残余的DMF, 在120 ℃下真空干燥12 h得到淡黄色粉末。
1.3 样品的表征
使用德国Bruker公司型号为D8 Advance的衍射仪测试粉末X射线衍射(XRD)图谱, 采用Cu靶Kα线(波长为0.1541 nm), 管电压设定为40 kV, 电流为40 mA, 扫描速率为2.4 (°)/min, 2θ扫描范围为2°~50°。在德国蔡司公司GeminiSEM 300扫描电镜(SEM)上观察样品形貌, 拍摄前对样品进行喷金处理, 电流为10 mA, 电压为15 kV。在美国ThermoFisher公司型号为Nicolet NEXUS670上测试样品的红外光谱(FT-IR)。采用美国Micromeritics公司ASAP2020全自动物理吸附仪在-196 ℃下测试样品的N2吸脱附曲线。在美国Radiant Technologies公司型号为Precision Multiferroic的铁电分析仪上测试样品的铁电性能。在美国ThermoFisher公司型号为Nicolet Evolution 500的紫外-可见光谱仪上测试样品的紫外-可见漫反射光谱(UV-Vis DRS)。样品测试前先用硫酸钡(BaSO4)做参比扣除背景, 光谱的波长扫描范围为200~800 nm。
1.4 机械催化降解染料性能测试
称取20 mg NH2-UiO-66粉末置于棕色反应瓶, 加入50 mL 5 mg/L的罗丹明B溶液。实验前, 先将溶液避光磁力搅拌1 h, 使催化剂与罗丹明B溶液之间充分吸附-脱附平衡, 然后在40、80、100 kHz(功率均为100 W)的超声波下进行机械催化实验, 每隔30 min取3 mL反应液用离心机离心过滤。取上清液, 用紫外-可见分光光度计(日本岛津UV2700)测试染料溶液的吸光度, 其中, 罗丹明B溶液的最大吸收峰为554 nm。根据公式D=(A0-At)/A0×100%计算溶液的降解率, 其中D为降解率, A0为吸附平衡后罗丹明B溶液在554 nm处的吸光度, At为超声振动t min后罗丹明B溶液在554 nm处的吸光度。对照实验: (a)罗丹明B溶液中加入20 mg NH2- UiO-66催化剂, 避光搅拌处理; (b)不加催化剂, 避光40 kHz超声波超声处理罗丹明B溶液; (c)在罗丹明B溶液中加入20 mg NH2-UiO-66催化剂, 同时加入10 mmol/L EDTA作为空穴捕获剂, 避光40 kHz超声波超声处理; (d)在罗丹明B溶液中加入20 mg NH2-UiO-66催化剂, 同时加入10 mmol/L BQ作为超氧自由基捕获剂, 避光40 kHz超声波超声处理; (e)在罗丹明B溶液中加入20 mg NH2-UiO-66催化剂, 同时加入10 mmol/L IPA作为羟基自由基捕获剂, 避光40 kHz超声波超声处理。
2 结果与讨论
2.1 催化剂的表征
合成的NH2-UiO-66的各项物化表征结果如 图1所示。样品的XRD特征衍射峰与晶体结构模拟的NH2-UiO-66的XRD衍射峰一致[40,41], 表明实验合成了结晶度较高的Zr基MOF材料(图1(a))。图1(b)为样品的FT-IR图谱, 由图可知NH2-UiO-66中羧基的配位方式为桥式配位(1380~1600 cm-1), 400~800 cm-1范围吸收峰对应MOF中O-Zr-O的振动吸收带。由样品的SEM照片可以看出制备的NH2- UiO-66呈大小均一的八面体结构(图1(c))。由样品的N2吸脱附等温曲线(图1(d))计算可以得到样品的比表面积高达780 cm2·g-1, 表明该类MOF材料具有高比表面积的特点。
图1
图1
NH2-UiO-66的表征图谱
Fig. 1
Characterizations of NH2-UiO-66
(a) XRD patterns; (b) FT-IR spectra; (c) SEM image; (d) N2 adsorption-desorption isotherm; (e) Ferroelectric hysteresis loop
2.2 机械催化性能
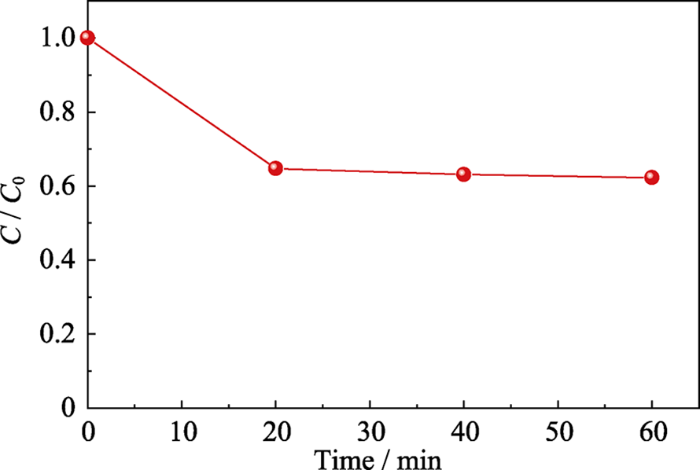
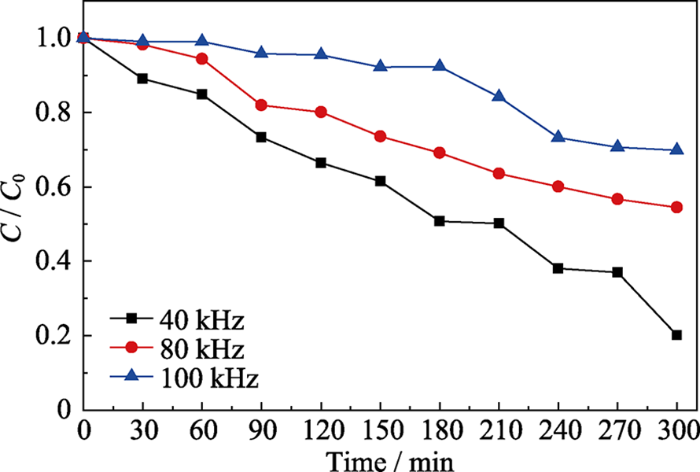
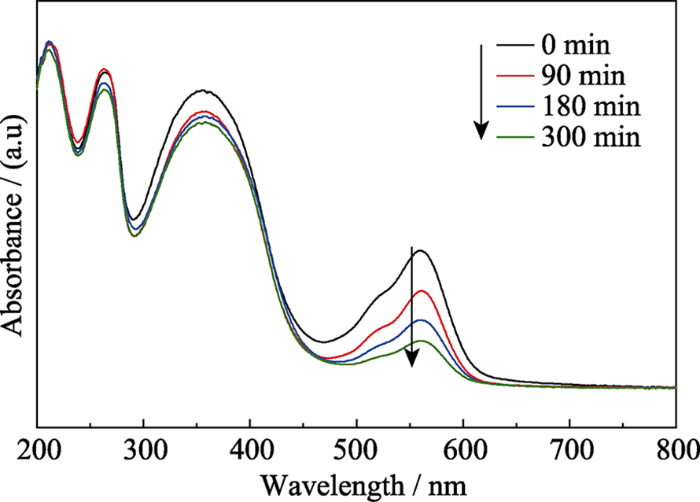
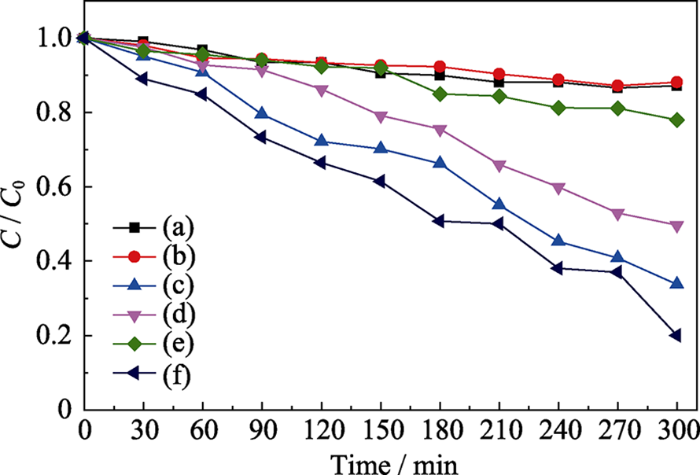
实验以50 mL质量浓度为5 mg/L的罗丹明B溶液作为模拟污染物, 考察NH2-UiO-66样品机械催化降解染料的性能。鉴于NH2-UiO-66具有高比表面积, 在催化过程中会吸附染料, 对其先进行吸附平衡实验。由图2可知, 在无机械振动的条件下, NH2-UiO-66样品吸附40 min后基本达到吸附平衡。吸附达到平衡之后, 在40、80及100 kHz的超声波作为机械源(功率为100 W)的作用下, 考察不同超声频率对NH2-UiO-66机械降解罗丹明B溶液的影响(图3)。结果表明随着超声频率的增大, 罗丹明B溶液的降解率反而下降, 其中超声频率为40 kHz时, 降解300 min后罗丹明B的降解率最高可达80%。这是由于较低的超声频率可产生较大的空化泡来增强超声空化作用, 而这些空化产生的机械力可作用到催化剂上, 使其表面弯曲产生较强的压电效应, 从而有利于染料的降解。取降解一定时间的催化剂过滤烘干, 采用固体粉末紫外-可见漫反射图谱来分析吸附在催化剂上罗丹明B随着降解时间的浓度变化关系, 结果如图4所示。360 nm处吸收峰对应催化剂NH2-UiO-66的吸收峰, 554 nm处吸收峰对应罗丹明B的最大吸收峰。随着超声振动时间的延长, 吸附在NH2-UiO-66表面的罗丹明B的最大吸收峰强度逐渐减弱, 表明罗丹明B逐渐被降解。MOF材料具有高比表面积, 其表面可大量吸附染料, 有利于染料的进一步降解。作为对照, 在只有催化剂无机械振动或只有机械振动无催化剂的条件下, 罗丹明B溶液紫外-吸收光谱基本没有变化(图5(a~b))。以上结果表明NH2-UiO-66具有机械催化降解罗丹明B的能力。
图2
图2
NH2-UiO-66吸附罗丹明B溶液平衡曲线图
Fig. 2
Equilibrium curve for the adsorption of RhB over NH2- UiO-66
图3
图3
不同超声波频率下NH2-UiO-66机械催化降解RhB溶液的结果
Fig. 3
Degradation results of RhB solution over NH2-UiO-66 under ultrasound with different frequencies
图4
图4
不同降解时间后催化剂的固体粉末紫外-可见漫反射图谱
Fig. 4
UV-Vis DRS of NH2-UiO-66 with different ultrasonic vibration time
图5
图5
不同条件下罗丹明B溶液的降解结果
Fig. 5
Degradation results of RhB solution under different conditions
(a) NH2-UiO-66 without vibration; (b) Vibration without catalyst; (c) Vibration+NH2-UiO-66+EDTA; (d) Vibration+NH2-UiO-66+BQ; (e) Vibration+NH2-UiO-66+IPA; (f) Vibration+NH2-UiO-66
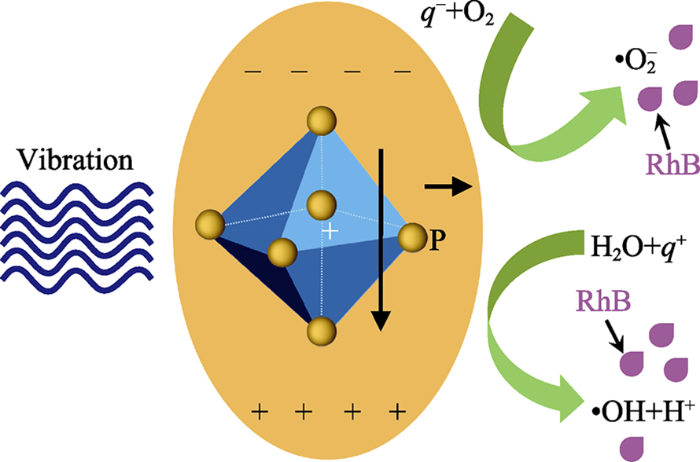
根据文献报道, 无机半导体铁电材料机械催化降解罗丹明B溶液过程中的主要活性物种是羟基自由基[43]。为了验证MOF材料机械催化过程的活性物种, 本研究开展了羟基自由基、超氧自由基和空穴的捕获实验, 结果如图5(c~e)所示。当体系加入乙二胺四乙酸二钠(EDTA)作为空穴捕获剂时, NH2-UiO- 66机械降解罗丹明B的效率没有发生明显的变化(图5(c)), 表明空穴在降解染料过程中基本不起作用。当体系加入对苯醌(BQ)作为超氧自由基捕获剂时, NH2-UiO-66机械降解罗丹明B的效率在一定程度上受到抑制(图5(d)), 表明超氧自由基在降解中起到一定的作用。而当体系加入异丙醇(IPA)作为羟基自由基捕获剂时, NH2-UiO-66对罗丹明B溶液的降解变得十分缓慢(图5(e)), 表明抑制剂可以有效地捕获机械振动时催化剂表面由正负电荷产生的具有强氧化性的羟基自由基, 阻止染料分子的降解。基于上述结果, 可以推测NH2-UiO-66机械催化降解罗丹明B的机理, 如图6所示。超声波对NH2- UiO-66施加机械振动, 由压电效应诱导NH2-UiO- 66纳米颗粒表面产生正负电荷, 产生的正-负电荷在内建电场的作用下分离到材料表面, 可以分别与染料溶液中的H2O和O2反应, 形成具有强氧化性的羟基自由基和超氧自由基, 将染料分子分解[15]。其化学反应过程可用如下反应式(1~4)表示。
图6
图6
NH2-UiO-66机械催化降解RhB机理示意图
Fig. 6
Schematic diagram for the mechano-catalytic degradation of RhB over NH2-UiO-66
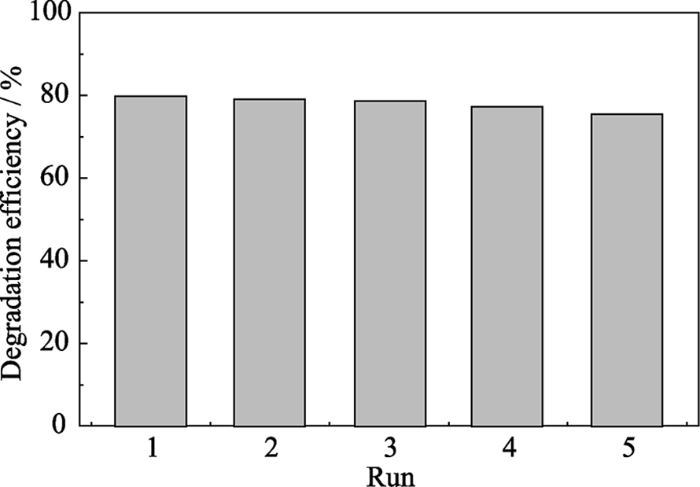
2.3 催化剂稳定性
图7
图7
NH2-UiO-66机械催化RhB循环利用5次实验结果
Fig. 7
Reusability of NH2-UiO-66 in the mechano-catalytic degradation of RhB
3 结论
采用溶剂热法成功制备了NH2-UiO-66, 其在降解罗丹明B中表现出良好的机械催化性能和稳定性, 5 h超声振动后罗丹明B的降解率可达80%。初步研究表明, Zr基MOF材料具有类似铁电材料的压电性能, 利用机械振动诱导其表面产生正负电荷, 进而形成具有强氧化活性的羟基自由基来分解染料分子。本研究对于拓展MOF材料的应用有着重要的指导意义, 同时也为开发利用机械催化技术处理染料废水提供了一种新的途径。
参考文献
Adsorptional photocatalytic degradation of methylene blue onto pectin-CuS nanocomposite under solar light
DOI:10.1016/j.jhazmat.2012.10.018
URL
PMID:23122730
[本文引用: 1]

This study describes the effect of adsorption on methylene blue degradation using pectin-CuS nanocomposite (PCSNC). The nanocomposite was synthesized using co-precipitation methods followed by direct encapsulation with pectin. The synthesized nanocomposite was characterized by SEM, TEM, XRD, FTIR and UV-vis spectral technique. The adsorption and photocatalytic efficiencies of PCSNC were compared with copper sulphide nanoparticle (CSNP). The dye removal was studied under different reaction conditions. The adsorption capacity of pectin based nanocomposite was higher due to other free functional group on pectin surface after connecting to nanoparticles. The simultaneous adsorption and photodegradation process (A+P) was the most efficient process due to rapid destruction of adsorbed dye molecules. The complete COD removal was attained in 10h using PCSNC/A+P process. On comparing with CSNP, pectin-CuS nano composite showed more degradation efficiency and reusability for MB degradation.
Pyroelectrically induced pyro-electro-chemical catalytic activity of BaTiO3 nanofibers under room-temperature cold-hot cycle excitations
Semiconductor-mediated photodegradation of pollutants under visible-light irradiation
Black lead molybdate nanoparticles: facile synthesis and photocatalytic properties responding to visible light
DOI:10.1016/j.apsusc.2014.12.068 URL [本文引用: 1]
Enhanced photocatalytic activity of hierarchical three dimensional metal oxide@CuO nanostructures towards the degradation of Congo Red dye under solar radiation
Synthesis, characterization, and photocatalytic properties of In2S3, ZnIn2S4, and CdIn2S4 nanocrystals
DOI:10.1021/acs.cgd.6b00050 URL [本文引用: 1]
Enhanced visible-light-driven photocatalytic disinfection performance and organic pollutant degradation activity of porous g-C3N4 nanosheets
DOI:10.1021/acsami.7b07657
URL
PMID:28758734
[本文引用: 1]

Porous g-C3N4 nanosheet (PCNS) photocatalyst with a thickness of 2.0 nm, pore volume of 0.61 cm(3) g(-1), and surface area of 190.1 m(2) g(-1) was prepared by a simple two-step template-free approach without the addition of extra reagents. Compared with the bulk g-C3N4 (BCN), PCNS possesses a greater number of surface reactive sites, improved efficiency of charge transfer, and accelerated separation of photogenerated electron-hole pairs. Accordingly, the visible-light-driven photocatalytic disinfection performance and organic pollutant degradation activity of PCNS are significantly enhanced. Escherichia coli (E. coli) cells can be killed completely by PCNS within 4 h, whereas only 77.1% of E. coli cells can be killed by BCN. The photodegradation rates of PCNS on methylene blue, Acid Red 27, and bisphenol A are almost 6.4, 4.0, and 1.9 times as fast as that of BCN, respectively. The photocurrent intensity of PCNS is about 3.7 times in comparison with that of BCN. Considering the easy preparation and excellent performance, PCNS could be a promising and competitive visible-light-driven photocatalyst in the field of environment remediation.
Hybridization of Cd0.2Zn0.8S with g-C3N4 nanosheets: a visible-light-driven photocatalyst for H2 evolution from water and degradation of organic pollutants
DOI:10.1039/c5dt01364j
URL
PMID:26200067
[本文引用: 1]

Novel visible-light-driven Cd0.2Zn0.8S/g-C3N4 inorganic-organic composite photocatalysts were synthesized by a facile hydrothermal method. The prepared Cd0.2Zn0.8S/g-C3N4 composites were characterized by X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS), Fourier transform infrared spectroscopy (FT-IR), transmission electron microscopy (TEM), high-resolution transmission electron microscopy (HRTEM), ultraviolet-visible diffuse reflection spectroscopy (DRS), photoluminescence (PL) spectroscopy and photoelectrochemical (PEC) experiments. Under visible-light irradiation, Cd0.2Zn0.8S/g-C3N4 photocatalysts displayed a higher photocatalytic activity than pure g-C3N4 and Cd0.2Zn0.8S for hydrogen evolution and degradation of pollutants, and the optimal g-C3N4 content was 20 wt%. The optimal composite showed a hydrogen evolution rate of 208.0 mumol h(-1). The enhancement of the photocatalytic activity should be attributed to the well-matched band structure and intimate contact interfaces between Cd0.2Zn0.8S and g-C3N4, which lead to the effective transfer and separation of the photogenerated charge carriers. Furthermore, the Cd0.2Zn0.8S/g-C3N4 photocatalysts showed excellent stability during photocatalytic hydrogen evolution and degradation of pollutants.
Photocatalytic degradation of 2,4,6-tribromophenol on Fe2O3 or FeOOH doped ZnIn2S4 heterostructure: insight into degradation mechanism
Mechano-catalytic overall water splitting
Strong vibration-catalysis of ZnO nanorods for dye wastewater decolorization via piezo-electro- chemical coupling
Piezoelectrochemical effect: A new mechanism for azo dye decolorization in aqueous solution through vibrating piezoelectric microfibers
Piezoelectrically induced mechano- catalytic effect for degradation of dye wastewater through vibrating Pb(Zr0.52Ti0.48)O3 fibers
Strong piezo-electrochemical effect of multiferroic BiFeO3 square micro-sheets for mechanocatalysis
DOI:10.1016/j.elecom.2017.04.017 URL [本文引用: 2]
Strong piezo-electro-chemical effect of piezoelectric BaTiO3 nanofibers for vibration-catalysis
Metal-organic framework materials as catalysts
DOI:10.1039/b807080f
URL
PMID:19384447
[本文引用: 1]

A critical review of the emerging field of MOF-based catalysis is presented. Discussed are examples of: (a) opportunistic catalysis with metal nodes, (b) designed catalysis with framework nodes, (c) catalysis by homogeneous catalysts incorporated as framework struts, (d) catalysis by MOF-encapsulated molecular species, (e) catalysis by metal-free organic struts or cavity modifiers, and (f) catalysis by MOF-encapsulated clusters (66 references).
Metal-organic frameworks for artificial photosynthesis and photocatalysis
A new three-dimensional bis(benzimidazole)-based cadmium(II) coordination polymer
Molecular simulations on CO2 adsorption and adsorptive separation in fullerene impregnated MOF-177, MOF-180 and MOF-200
A systematic evaluation of UiO-66 metal organic framework for CO2/N2 separation
DOI:10.1016/j.seppur.2019.04.081 URL [本文引用: 1]
Amino-silane-grafted NH2-MIL-53(Al)/polyethersulfone mixed matrix membranes for CO2/CH4 separation
A novel peroxidase mimetic Co-MOF enhanced luminol chemiluminescence and its application in glucose sensing
DOI:10.1016/j.snb.2019.126631 URL [本文引用: 1]
A water-stable La-MOF with high fluorescence sensing and supercapacitive performances
Fabrication of a water-stable luminescent MOF with an open Lewis basic triazolyl group for the high-performance sensing of acetone and Fe3+ ions
DOI:10.1007/s10853-019-03638-x URL [本文引用: 1]
Semiconductor behavior of a metal-organic framework (MOF)
DOI:10.1002/chem.200601003
URL
PMID:17385196
[本文引用: 1]

Upon light excitation MOF-5 behaves as a semiconductor and undergoes charge separation (electrons and holes) decaying in the microsecond time scale. The actual conduction band energy value was estimated to be 0.2 V versus NHE with a band gap of 3.4 eV. Photoinduced electron transfer processes to viologen generates the corresponding viologen radical cation, while holes of MOF-5 oxidizes N,N,N',N'-tetramethyl-p-phenylenediamine. One application investigated for MOF-5 as a semiconductor has been the shape-selective photocatalyzed degradation of phenol in aqueous solutions.
Zirconium metal-organic framework supported highly-dispersed nanosized BiVO4 for enhanced visible-light photocatalytic applications
Synthesis of Zr-based MOF nanocomposites for efficient visible-light photocatalytic degradation of contaminants
Ligand modification of UiO-66 with an unusual visible light photocatalytic behavior for RhB degradation
DOI:10.1039/c7dt04477a
URL
PMID:29340397
[本文引用: 1]

A series of isostructural UiO-66-X (X = H, NH2, Br, (OH)2, (SH)2) catalysts have been successfully synthesized by modifying different functional groups on the ligand. The effects of the ligand modification of UiO-66 were investigated for their photocatalytic activity of Rhodamine B degradation under visible light. Surprisingly, UiO-66-NH2 and UiO-66-(OH)2 which have narrow bandgaps and excellent visible light absorption do not show outstanding photocatalytic performances compared to UiO-66 and UiO-66-Br. Electrochemical test results indicated that the conduction band potential of UiO-66-X and the separation efficiency of electrons were quite important in these photocatalytic reactions, other than the electronic effect as reported. Similar photocatalytic degradation behaviors were found for Congo red and methyl orange. Herein, we firstly reported different mechanisms of selective degradation in the case of UiO-66, which subverted the previous understanding of photodegradation behavior.
UiO-66(Zr) coupled with Bi2MoO6 as photocatalyst for visible-light promoted dye degradation
Effective electron-hole separation over a controllably constructed WP/UiO-66/CdS heterojunction to achieve efficiently improved visible-light-driven photocatalytic hydrogen evolution
DOI:10.1039/c9cp01180c
URL
PMID:30964138
[本文引用: 1]

The photocatalytic decomposition of water to produce hydrogen is an important strategy to effectively utilize solar energy and solve the energy crisis. In this study, a highly efficient WP-nanoparticle-modified composite catalyst was successfully prepared. WP nanoparticles have been used as an efficient and acid-stable co-catalyst for the HER owing to their specific electronic structure, metalloid characteristics and catalytic activity. On the one hand, the octahedral spatial structure of UiO-66 not only provides attachment space for CdS and WP nanoparticles, but also effectively reduces the particle size and increases the dispersion of CdS and WP nanoparticles. On the other hand, the potential difference and the matching energy band positions of UiO-66 and CdS provide a feasible thermodynamic path for the transmission of photogenerated electrons. The intimate contact between the abovementioned three compounds resulted in a strong synergistic effect, which improved the efficiency of the photocatalytic H2 production. Under visible-light irradiation, the maximum H2 production in 5 h over the [UiO-66@CdS/WP (10 wt%)] photocatalyst was 395 mumol, which was 26.33 times that of pure CdS. The physical and chemical information of the samples could be obtained through XRD, SEM, TEM, XPS, BET and UV-vis DRS characterizations. Furthermore, based on the photoluminescence spectra, photoelectrochemical experiments and Mott-Schottky curves, we could reasonably explain the separation and transfer mechanisms of the photogenerated electrons and holes. The lower recombination rate of charge, enhanced intensity of light absorption, a short fluorescence lifetime (2.11 ns), a faster electron injection rate (KET = 2.32 x 108 s-1), a larger efficiency of electron injection (etainj = 49.1%), high photocurrent response, and smaller charge transfer resistance accelerate the efficient separation and transfer of spatial charges, finally enhancing the photocatalytic performance.
Orderly-designed Ni2P nanoparticles on g-C3N4 and UiO-66 for efficient solar water splitting
DOI:10.1016/j.jcis.2018.07.138
URL
PMID:30096523
[本文引用: 1]

Stable and efficient photocatalyst is the key important research goals up to now. On account of the dominant performance of Ni2P, g-C3N4 (graphitized carbonitride) and UiO-66 (Universitetet i Oslo) themselves, an orderly-designed assemble of g-C3N4/UiO-66/Ni2P is successfully designed and assembled with capability of high-efficient dye-sensitized photocatalytic H2 evolution. The electron transport routes are successfully adjusted and the hydrogen evolution is greatly improved. It exhibits synergistic effect on highly efficient photocatalytic hydrogen production. The maximum amount of hydrogen evolution reaches about 200mumol for 5h over the g-C3N4/UiO-66/Ni2P photocatalyst under the 5W LED white light at 420nm. The H2 evolution rate is 12 times high than over g-C3N4. Such synergistically increased effect in photocatalytic properties is certified by related characterization results such as TEM, SEM, XPS, XRD, UV-vis DRS, Transient photocurrent and FT-IR etc. The above studies show that the Ni2P nanoparticles modified on the g-C3N4/UiO-66 provides the more active sites and improves the efficiency of photo-generated charge separation. In addition, the possible mechanism of photocatalytic hydrogen production is proposed.
Zr-MOFs based on keggin-type polyoxometalates for photocatalytic hydrogen production
DOI:10.1007/s10853-018-2476-0 URL [本文引用: 1]
Hydrogen production with ultrahigh efficiency under visible light by graphene well-wrapped UiO-66- NH2 octahedrons
Amino-assisted NH2-UiO- 66 anchored on porous g-C3N4 for enhanced visible-light-driven CO2 reduction
DOI:10.1021/acsami.9b04302
URL
PMID:31373194
[本文引用: 1]

Constructing heterostructured photocatalysts is an efficient method to improve photocatalytic carbon dioxide (CO2) reduction. Herein, holey g-C3N4 (HGN) with rich amino groups (-NHx) was hybridized with NH2-UiO-66 (NUZ) via a facile in situ growth method. NUZ nanocrystals were anchored on HGN via NHx-Zr-O chemical bonding, leading to the uniform dispersion and avoiding the leaching of NUZ, thus showing excellent stability in photocatalysis. The chemically bonded interfacial charge transfer effect originated from the NHx-Zr-O formation efficiently accelerated the separation and migration of charge carriers, improving the photoactivity. Benefiting from the NHx-Zr-O formation, the optimized NUZ/HGN-35% heterojunctions exhibited outstanding activity in the photoreduction of CO2 to CO (31.6 mumol g(-1) h(-1)), which was about 2 and 3 times higher than that of pure NUZ and HGN under visible-light irradiation. This study is expected to provide useful insights for constructing composites with strong interaction for CO2 reduction, H2 production, and N2 reduction.
N-CND modified NH2-UiO- 66 for photocatalytic CO2 conversion under visible light by a photo- induced electron transfer process
Ru nanoclusters supported on HfO2@CN derived from NH2-UiO-66(Hf) as stable catalysts for the hydrogenation of levulinic acid to γ-valerolactone
DOI:10.1016/j.catcom.2019.105710 URL [本文引用: 1]
Pd nanoparticles encaged within amine-functionalized metal-organic frameworks: catalytic activity and reaction mechanism in the hydrogenation of 2,3,5- trimethylbenzoquinone
Enhanced sonocatalytic degradation of carbamazepine and salicylic acid using a metal-organic framework
DOI:10.1016/j.ultsonch.2019.04.019
URL
PMID:31101253
[本文引用: 1]

A metal-organic framework (MOF) was used as a sonocatalyst for ultrasonic (US) processes, to improve the degradation of two selected pharmaceutical active compounds (PhACs); carbamazepine (CBM) and salicylic acid (SA). The intrinsic characteristics of the MOF were characterized using a porosimeter (N2-BET) and scanning electron microscope (SEM). Various experiments were carried out under conditions with different US frequencies (28 and 1000kHz), US power densities (45-180WL(-1)), pH conditions (3.5, 7, and 10.5), and temperatures (293, 303, and 313K) to investigate the degradation rates of the selected PhACs. Improved removal rates of PhACs were demonstrated within 60min at 28kHz (46% for SA; 47% for CBM) and 1000kHz (60% for SA; 99% for CBM) with an MOF concentration of 45mgL(-1) in the US/MOF system, in comparison to 28kHz (20% for SA; 25% for CBM) and 1000kHz (37% for SA; 97% for CBM) under the 'US only' process. The removal of CBM was greater than that of SA under all experimental conditions due to the intrinsic properties of the PhACs. The degradation rates of PhACs are related to the quantity of H2O2; degradation is thus mostly affected by OH oxidation, which is generated by the dissociation of water molecules. The advantages of the 'US/MOF system' are as follows: (i) dispersion of MOF by US can improve sites and reactivity with respect to adsorption between the adsorbate (PhACs) and the adsorbent (MOF), and (ii) dispersed MOF acted as additional nuclei for water molecule pyrolysis, leading to the production of more OH. Therefore, based on the synergy indices, which were calculated using the removal rate constants [k1 (min(-1))] of the pseudo-first order kinetic model, the 'US/MOF system' can potentially be used to treat organic pollutants (e.g., PhACs).
Design of the hybrid metal- organic frameworks as potential supramolecular piezo-/ferroelectrics
DOI:10.1021/acs.jpcc.8b08442 URL [本文引用: 1]
Microwave-assisted synthesis of well-shaped UiO-66-NH2 with high CO2 adsorption capacity
Facile synthesis of amine-functionalized UiO-66 by microwave method and application for methylene blue adsorption
Piezoelectric and ferroelectric materials and structures for energy harvesting applications
Microwave synthesis of ZnxCd1-xS nanorods and their photocatalytic activity under visible light