水热法合成锌空气电池氧还原二氧化锰催化剂

Preparation of Manganese Dioxide for Oxygen Reduction in Zinc Air Battery by Hydro thermal Method
a1: #cod#x003b4;-MnO 2 ; b1:#cod#x003b1;-MnO 2 ; c1:#cod#x003b2;-MnO 2 2.3 TG analysis The results of TG analysis of the as-prepared samples are shown in Fig. 3. From the TG profiles, it can be seen that the samples show different thermal behavior. Two major weight losses occur between 50℃ and 280℃. The first weight loss 1.65wt%, 1.73wt% and 1.62wt% for a1, b1 and c1, respectively below 120℃ can be attributed to desorption of physisorbed water whilst the release of chemisorbed water may be responsible for the significant weight loss 6.43wt%, 6.08wt% and 4.95wt% for a1, b1 and c1, respectively occurred between 120 and 280℃. Fig. 3 TG analysis for the as-prepared samples
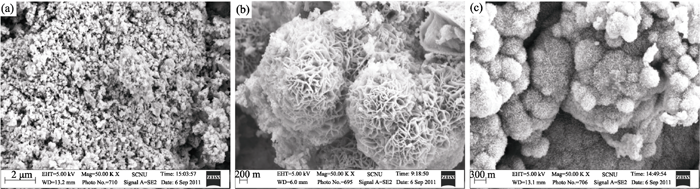
a1:#cod#x003b4;-MnO 2 ; b1:#cod#x003b1;-MnO 2 ; c1:#cod#x003b2;-MnO 2 2.4 SEM analysis and BET specific surface area The morphology of the as-prepared samples was ob- served by SEM, as shown in Fig. 4. From Fig. 4a, it can be seen that the product particles are uniformly distributed and the products are mainly composed of spherical-like particles with 250 nm of average grain size. For the active material, the smaller particles have larger specific surface area and shorter diffusion distance, which provides better rate performance. Fig. 4b shows the existence of the opening hydrangea like morphologies and the nanostructued particles in the as-prepared b1 sample about 500 nm. Figure 4c shows that there exists a great deal of large particles of MnO 2 , which consisted of aggregation of MnO 2 hystrichosphere particles about 250 nm. Thus, the morphology can be affected by the concentrations of the reactants. However, the actual mechanism is not clear for the moment and needs to be further investigated. Fig. 4 SEM images of the as-prepared samples a a1#cod#x003b4;-MnO 2 ; b b1#cod#x003b1;-MnO 2 ; c c1#cod#x003b2;-MnO 2