|
|
Inorganic Environmental Materials and Their Applications in Pollutant Removal
SHI Weiqun, WANG Xiangke
2020 Vol. 35 (3): 257–259
 Abstract
Abstract(
1000 )
 HTML
HTML(
65)
 PDF
PDF(252KB)(
1123
)
With the fast advancement of human modernization and rapid development of social economy, traditional energy consumption has been enormously increased and climate change has therefore become a very challenging issue for the human being. The development of modern industry, especially the chemical industry, has brought not only convenience to people, but also unprecedented destruction to the ecological environment closely related to human life. Energy and environmental issues have become a global challenge for us today. In order to better deal with the challenges and protect our living homeland, the majority of researchers are constantly seeking and exploring new materials and technologies which are environmentally friendly and can be used efficiently, aiming to solve increasingly serious environmental problems. In current stage, environmental materials and technologies have received ever-increasing attention and are developing rapidly. Environmental materials, as the name implies, are materials designed and developed for environmental issues. The key issue of environmental problems is environmental pollution. At present, the widely concerned pollutants include gas pollutants, persistent organic pollutants (POPs), and heavy metals. At the same time, with drastic development of nuclear energy industry in the past two decades in China, radioactive pollutants have also received increasing attention. Separation and removal of these pollutants from environment by certain means is an effective and common method for environmental pollution control. Therefore, the key for solving environmental problems is to develop materials and technologies that can effectively remove environmental pollutants. Plenty of versatile materials for specific contaminant removals have been reported over the past few decades. These materials come in a wide variety of functions, with varying structures and performance. Most concerned materials include traditional molecular sieves[1], mineral materials[2], carbon materials such as graphene and carbon nanotubes[3], polymer based materials such as resins[4], metal organic frameworks (MOFs)[5] and covalent organic frameworks (COFs)[6]. Among these materials, inorganic materials have broad application prospects in the removal and separation of environmental pollutants due to their stability, low cost and environmental friendliness. In particular, inorganic nanoporous materials have become favorable in recent years. The nanometer size makes nanomaterials not only have quantum size effect, but also possess larger specific surface area and more surface atoms compared to other common materials, thus exhibiting stronger adsorption ability and better dispersibility in aqueous solution. In addition, the porosity of materials greatly enhances the specific surface area and correspondingly increases the contact opportunity between the material and contaminant. Meanwhile, it also improves the diffusion and transportation of contaminants inside the material, elevating the adsorption kinetics. Metal nanomaterials, metal oxide nanomaterials, mineral materials, etc. are typical representatives of inorganic nanomaterial family. Based on the published works, many efforts in the field of inorganic environmental materials focused on improving the removal efficiency and selectivity toward one or more target pollutants. Inorganic materials have higher stability than organic materials, but possess low removal capacity and poor selectivity, due to lacking of active functional groups on the surface. Functionalization of inorganic materials should be a reasonable approach to overcome this drawback. It is well known that functional groups with strong binding or coordination ability to the target contaminant decorated on the surface of material by physical or chemical means can greatly improve the adsorption performance of inorganic materials[7]. Moreover, besides modifying the specific recognition group on the surface of the material[8], adjusting the pore structure of material and physically screening contaminants by the size effect[9] are always common and effective. It is also promising to combine size effects, bonding and electrostatic interactions by means of molecular imprinting or composition[10]. Besides, developing inorganic environmental materials used under harsh conditions[11], such as high acid, high alkali, and high temperature, is becoming a research hot topic in recent years. In all, after decades of development, researches on inorganic environmental materials have made significant progress, whereas most of materials are still not satisfactory for industrial applications. In order to better solve the increasingly serious environmental problems, it is still necessary for the material researchers to overcome difficulties and make continuous efforts.

|
|
|
Recent Advances in Carbon Nitride-based Nanomaterials for the Removal of Heavy Metal Ions from Aqueous Solution
WANG Xiangxue, LI Xing, WANG Jiaqi, ZHU Hongtao
2020 Vol. 35 (3): 260–270
 Abstract
Abstract(
1523 )
 HTML
HTML(
64)
 PDF
PDF(3781KB)(
1533
)
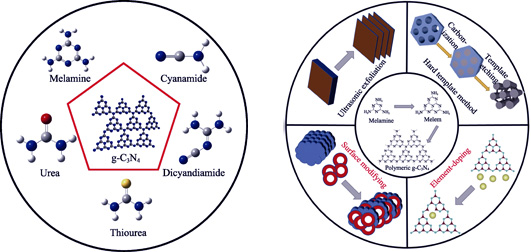
Graphitic-like carbon nitride (g-C3N4), one of the most significant two-dimensional layered materials, has attracted worldwide attention in multidisciplinary areas such as photocatalysis, energy conversion and environmental pollution management. Its derivative compounds have also attracted multifarious attention owing to the intrinsic characters of their stable physicochemical properties, low cost and environmentally friendly features. This review focus on the design of high-performance g-C3N4-based nanomaterials and their potential for pollutant elimination in environmental pollution cleanup. Over the past few years, signi?cant advances have been achieved to synthesize g-C3N4 and g-C3N4-based nanomaterials, and their properties have been enhanced and characterized in detail. In this review, recent developments in the synthesis and modification of g-C3N4-based nanomaterials are summarized. The applications in heavy metal ions adsorption from wastewaters are gathered and their underlying reaction mechanisms are discussed. Finally, a summary and outlook are also briefly illustrated.
|
|
|
Ceramic Solidification of Salt-containing Waste from Pyrochemical Reprocessing of Spent Nuclear Fuel
LIU Yalan, CHAI Zhifang, SHI Weiqun
2020 Vol. 35 (3): 271–276
 Abstract
Abstract(
666 )
 HTML
HTML(
17)
 PDF
PDF(1315KB)(
834
)
For the future advanced nuclear fuel cycle system, pyrochemical technology based on molten salt electrolysis is generally considered to be one of the most promising and reliable reprocessing technologies. The salt-containing waste generated in each step of the pyrochemical process needs to be converted into a ceramic waste form, which can be stably disposed in a long term manner in deep geological repository. This is of pivotal importance for the scale-up and industrialization of molten salt electrolysis based pyrochemical processing. In this review, the current research progresses of ceramic solidification technology in main nuclear energy countries with respect to salt-containing wastes were summarized and reviewed, with emphasis on ceramic solidifications of salt-containing wastes from electro-reduction process in LiCl-based salt and electro-refining process in LiCl-KCl salt. In addition, future perspectives in this field are also given.
|
|
|
Adsorption of Phenolic Organic Pollutants on Graphene Oxide: Molecular Dynamics Study
ZHAO Chaofeng, JIN Jiaren, HUO Yingzhong, SUN Lu, AI Yuejie
2020 Vol. 35 (3): 277–283
 Abstract
Abstract(
913 )
 HTML
HTML(
52)
 PDF
PDF(2821KB)(
1145
)
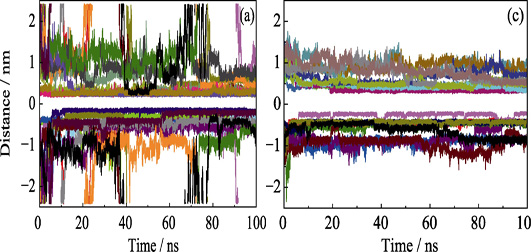
In this work, molecular dynamics (MD) simulations were applied to address the major concerns about the independent and competitive adsorption processes of phenolic organic pollutants (POPs) on the graphene oxide (GO) in aqueous solution. Phenol, α-naphthol and 4-octyl-phenol were adopted as representatives of POPs and their adsorption energies were calculated, which followed an order of 4-octyl-phenol (41.34 kJ/mol)>α-naphthol (33.23 kJ/mol)> phenol (19.31 kJ/mol). The simulation results showed that hydrophobic properties of POPs were recognized as the driving force for their adsorption behaviors. Moreover, van der Waals interaction, electrostatic interaction, as well as hydrogen bonds, may also improve the adsorption capacity of GO towards POPs. The competitive adsorption process revealed that in addition to the direct adsorption onto the GO surface, the molecular aggregation may be another indirect adsorption way existed in the mixed system. Understanding the interaction between GO and POPs in aqueous solution is critical to the design and application of graphene-based materials and our findings are believed to contribute further theoretical basis to the engineering treatment of POPs-containing waste water.
|
|
|
Distinguished Cd(II) Capture with Rapid and Superior Ability using Porous Hexagonal Boron Nitride: Kinetic and Thermodynamic Aspects
LI Li, GUO Xiaojie, JIN Yang, CHEN Chaogui, Abdullah M Asiri, Hadi M Marwani, ZHAO Qingzhou, SHENG Guodong
2020 Vol. 35 (3): 284–292
 Abstract
Abstract(
638 )
 HTML
HTML(
18)
 PDF
PDF(1550KB)(
907
)
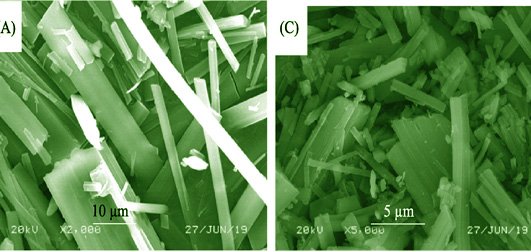
In present work, a systematical and comprehensive understanding for the adsorption of Cd(II) on porous hexagonal boron nitride (p-BN) was studied. The chemical compositions, morphology and surface functional groups of p-BN before and after adsorption were characterized by SEM, HRTEM, BET, XRD, and FT-IR. The effects of pH, adsorbent dosage, contact time and temperature on Cd(II) adsorption were investigated. The maximum adsorption capacity for Cd(II) achieves 184 mg·g -1 at pH 7.0 and 313 K. The kinetic data fitted well with pseudo-second-order model and intra-particle diffusion model, indicating that the adsorption is mainly controlled by chemisorption, and the rate-limiting step is the molecular diffusion. The adsorption isotherms are in accordance with Freundlich and Langmuir model respectively, suggesting Cd(II) adsorbed on the heterogeneous surface through multilayer and monolayer adsorption. The thermodynamic parameters are calculated to confirm the spontaneous and endothermic process of Cd(II) sorption. Spectroscopic results from XPS imply that p-BN adsorbent had substantial functional groups and bonding sites, which is propitious to uptake Cd(II) from wastewater. These results revealed that p-BN is a promising candidate for Cd(II) scavenging.
|
|
|
Microscopic Insights into pH-dependent Adsorption of Cd(II) on Molybdenum Disulfide Nanosheets
DONG Lijia, GUO Xiaojie, LI Xue, CHEN Chaogui, JIN Yang, AHMED Alsaedi, TASAWAR Hayat, ZHAO Qingzhou, SHENG Guodong
2020 Vol. 35 (3): 293–300
 Abstract
Abstract(
578 )
 HTML
HTML(
12)
 PDF
PDF(1737KB)(
627
)
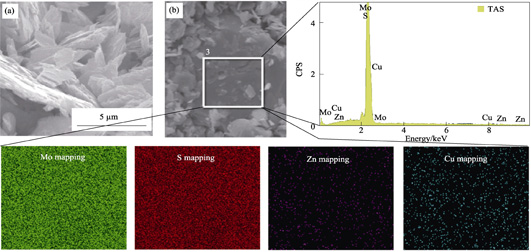
Herein, the retention mechanisms and microstructure of Cd(II) on MoS2 nanosheets were evaluated by batch experiments and EXAFS technology. The sorption of Cd(II) on MoS2 was strongly affected by solution pH, contact time, and temperature, but not by the ionic strength. The solution pH could only promote the sorption capacity, but does not improve the sorption rates and change the sorption isotherms and thermodynamics in the pH range of 3.3-9.6. The pseudo-second-order model could fit the equilibrium data better and the intra-particle diffusion model showed three typical stages in the sorption process. The isotherms and thermodynamics analysis indicated that the heterogeneity sorption of Cd(II) onto MoS2 was a spontaneous, endothermic, and irreversible process. The EXAFS spectra revealed the coexistence of two sorption types. The inner-sphere complexation was formed in the form of Cd-S bond at lower pH (3.56, 6.48), while the Cd(OH)2 precipitation occurred in the form of Cd-O and Cd-Cd bonds at higher pH (9.57). These results provide new insights into the interaction mechanisms between metal ions and MoS2 nanosheets.
|
|
|
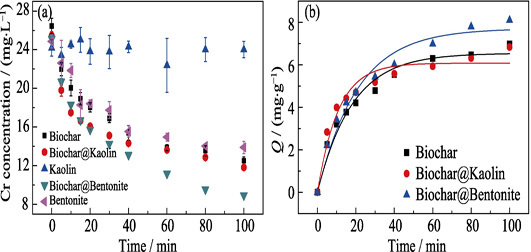
Adsorption of Cr(VI) from Aqueous Solution by Biochar-clay Derived from Clay and Peanut Shell
WANG Hai, YANG Ningcan, QIU Muqing
2020 Vol. 35 (3): 301–308
 Abstract
Abstract(
1140 )
 HTML
HTML(
46)
 PDF
PDF(1635KB)(
1244
)
Heavy metal chromium pollution seriously threatens the environmental safety of soil and water body. The Cr(VI) compound has strong migration ability, enrichment and strong oxidizing ability. These properties make Cr(VI) ions more dangerous and difficult to handle. Adsorption technology is a simple and effective method for treatment of heavy metal pollution. The two clay-biochars are made by mixing biochar derived from peanut shell and two kinds of clays (Kaolin and bentonite) under magnetic stirring conditions. A variety of characterizations suggest that clays uniformly deposit on the surface of biochar. Adsorption experiments indicate that the sorption of Cr(VI) ions from wastewater on Kaolin-biochar is significantly higher than that of bentonite-biochar. Adsorption kinetic of Cr (VI) on two clay-biochars follows satisfactorily the pseudo-second order model due to high correlation coefficient (R2>0.999). Adsorption isotherms of Cr(VI) on Biochar@Bentonite are fitted by Langmuir model, whereas the Freundlich model fits better the Cr(VI) sorption on Biochar@Kaolin. These findings are crucial for the potential application of clay-biochar composites for the treatment of the immobilization of heavy metals in environmental remediation.
|
|
|
C@K2Ti6O13 Hierarchical Nano Materials: Effective Adsorption Removal of Cr(VI)
ZHU Mingyu, FAN Dezhe, LIU Bei, LIU Shuya, FANG Ming, TAN Xiaoli
2020 Vol. 35 (3): 309–314
 Abstract
Abstract(
464 )
 HTML
HTML(
8)
 PDF
PDF(1134KB)(
729
)
Cr(VI) is toxic to the organic life. Eliminating the pollutant of Cr(VI) in solution has become a hot research field. In this work, the C@K2Ti6O13 hierarchical nanomaterials were prepared by liquid phase deposition combined with hydrothermal treatment, which were constituted by carbon nano spheres and K2Ti6O13 nanobelts. The morphology and phase of the materials were characterized by different methods. The C@K2Ti6O13 hierarchical nanomaterials show high potential as adsorbent to adsorb Cr(VI). Effect of initial pH, adsorption time and ionic strength on Cr(VI) adsorption by C@K2Ti6O13 composite nanostructures were investigated. The elimination rate of Cr(VI) can reach 50% in 1 h. The adsorption kinetics agrees well with the pseudo- second-order kinetic model, and the adsorption thermodynamics accords with the Langmuir isotherm adsorption model. It can be expected that the C@K2Ti6O13 hierarchical nanomaterials have great application potential in environmental treatment.
|
|
|
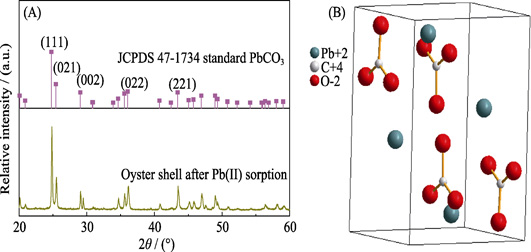
High-efficiency Biogenic Calcium Carbonate for Adsorption of Pb(II) and Methyl Orange from Wastewater
DU Xudong, TANG Chengyuan, YANG Xiaoli, CHENG Jianbo, JIA Yuke, YANG Shubin
2020 Vol. 35 (3): 315–323
 Abstract
Abstract(
697 )
 HTML
HTML(
17)
 PDF
PDF(1300KB)(
770
)
A low-cost oyster shell was carried to prepare biogenic calcium carbonate (bio-CaCO3) to remediate Pb(II) and methyl orange (MO) from contaminated water. The morphology, composition and structure of the material were analyzed mainly by scanning electron microscope (SEM), thermogravimetric analysis (TGA), X-ray fluorescence (XRF). The adsorption of Pb(II) and MO by bio-CaCO3 was studied by combining batch experiments and microstructure characterization. Batch sorption experiments showed that 45% MO was removed by bio-CaCO3 (msorbent/Vsolvent=0.2 g/L, [MO]initial=60 mg/L). An obviously morphology change took place after MO adsorbed onto bio-CaCO3. The maximum sorption capacity of bio-CaCO3 for Pb(II) is 1775 mg/g (pH=5.0, T=298 K), which is higher than that of the traditional nanomaterials such as bentonite and activated carbon. The Pb(II) removal mechanism is expected to be CaCO3+ Pb(II)→PbCO3, where the ΔH θ, ΔS θ and ΔG θ of Pb(II) sorption by bio-CaCO3 (pH=5.0, T=298K) are -7.64 kJ/mol, -17.92 J/(mol·K) and -2.30 kJ/mol, respectively. More regular products with quadrangular structure are formed after Pb(II) adsorption. The results highlight that the bio-CaCO3 has a high Pb(II) and MO sorption efficiency, demonstrating that it is a promising adsorbent material in environmental pollution management.
|
|
|
Mechanism Study of Tetracycline High Efficient Photodegradation by Bi2WO6 Nanosheets under Visible Light Irradiation
WEI Xin, LU Zhanhui, WANG Luping, FANG Ming
2020 Vol. 35 (3): 324–328
 Abstract
Abstract(
564 )
 HTML
HTML(
18)
 PDF
PDF(973KB)(
928
)
As a narrow band gap semiconductor, Bi2WO6 has great application potential in photo-degradation of organic pollutants, such as tetracycline. In present work, Bi2WO6 nanosheets were successfully synthesized by a hydrothermal method and the photo-degradation of tetracycline under visible light irradiation were investigated. XRD, FESEM, TEM and absorption spectra were used to characterize the structure and morphology of the material. It was found that when adding 50 mg Bi2WO6 nanosheets into 50 mL of tetracycline solution at pH=8, 85% tetracycline (50 mL, 50 mg/L) was photodegraded within 130 min. The photoelectron-chemical experiments and free radical capture experiments were performed to explore the photo-degradation mechanism. The results show that good photocatalytic performance of Bi2WO6 nanosheets are ascribed to the high electron density and photoelectron-hole separation efficiency.
|
|
|
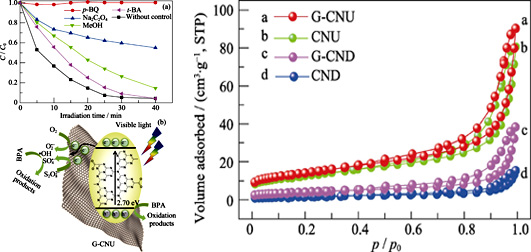
Visible-light-driven Activation of Persulfate by RGO/g-C3N4 Composites for Degradation of BPA in Wastewater
ZHANG Sai, ZOU Yingtong, CHEN Zhongshen, LI Bingfeng, GU Pengcheng, WEN Tao
2020 Vol. 35 (3): 329–336
 Abstract
Abstract(
828 )
 HTML
HTML(
21)
 PDF
PDF(2478KB)(
1065
)
Persistent organic pollutants can be effectively removed by photocatalytic oxidation, which reveals potential application prospects in the field of wastewater purification. Binary reduced graphene oxide and graphitic carbon nitride (RGO/g-C3N4) visible-light photocatalyst was successfully fabricated by the freeze drying assisted thermal polymerization method with urea and dicyandiamide as raw materials, respectively. The morphologies, structures and optical properties were characterized by various techniques. It was found that g-C3N4 and RGO nanostructures formed an intimate contact across the heterojunction interface. The photodegradation performances of catalysts were evaluated by removing bisphenol A (BPA) with the activation of persulfate (PS). The results indicated that the photocatalytic activities of photocatalysts were enhanced with the addition of PS as an oxidant and electron acceptor under visible light irradiation (λ>420 nm). Moreover, BPA was almost completely removed by RGO/g-C3N4 prepared with urea as raw material in 40 min. After five recovery tests, the removal efficiency of BPA for the catalyst was up to 80% within 40 min under visible light irradiation, which exhibited superior stability.
|
|
|
Removal of Boron from Water by Mg-Al-Ce Hydrotalcite Adsorption
ZHANG Wei, LIU Chen, CHEN Yuantao, WU Wangsuo
2020 Vol. 35 (3): 337–344
 Abstract
Abstract(
593 )
 HTML
HTML(
21)
 PDF
PDF(4894KB)(
804
)
Boron is an important micronutrient for plants, animals, and humans. However, high concentrations of boron are harmful to animals and plants. A magnesium-aluminum-cerium hydrotalcite (Mg-Al-Ce-HT) was successfully prepared by the co-precipitation method for boron removal. Different analyses were conducted to confirm the structure and characteristics of Mg-Al-Ce-HT. Adsorption efficiency of Mg-Al-Ce-HT was studied as a function of initial pH, amount of adsorbent, concentration of initial boric acid, and contact time. The pH of the solution had a negligible effect on boron sorption when pH was less than 8.0. However, the adsorption capacity decreased when the pH exceeded 8.0. The optimum amount of the adsorbent was 200 mg, and the maximum adsorption capacity was 32.52 mg·g -1. Boron removal reached equilibrium at 160 min. The thermodynamic parameters revealed that the adsorption was a non-spontaneous and endothermic process. The data fitted well with the Langmuir model, which indicated that the process involved monolayer adsorption.
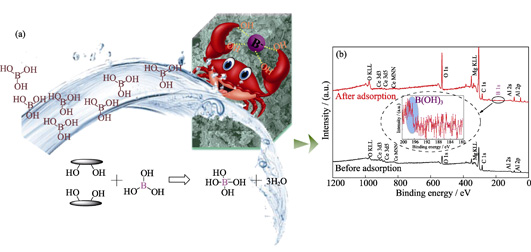
|
|
|
Adsorption of Iodine by ZIF Materials
LIU Rong, ZHANG Wei, CHEN Yuantao, FAN Yuanrui, HU Guangzhang, XU Cheng, HAN Zheng
2020 Vol. 35 (3): 345–351
 Abstract
Abstract(
1235 )
 HTML
HTML(
54)
 PDF
PDF(5968KB)(
1189
)
Radioactive iodine present in nuclear waste water streams is harmful to human health and environment. Since iodine will exist in multiple states in water, accurate quantification of the total iodine content in any given sample is very difficult. The development of a method for determining the total iodine content accurately in water, and finding materials which can effectively remove the iodine are of particular importance. Here, a method was proposed for determining the concentration of iodine by cyclohexane extraction. And two kinds of zeolite imidazole skeleton materials ZIF-8 and ZIF-67 were prepared to be used as adsorbents to effectively adsorb iodine from an aqueous environment. Samples ZIF-8 and ZIF-67 were characterized by different methods. The results show that these two kinds of materials have good chemical structure and large specific surface area. The results of adsorption kinetics experiments show that the adsorption of ZIF-8 and ZIF-67 materials to iodine can reach the equilibrium within 60 min. The iodine adsorption behaviors of both materials are fitted with the pseudo second-order kinetic model. Their adsorption thermodynamics indicate linear adsorption behavior for iodine in the case of both zeolites. Adsorption capacities of ZIF-8 and ZIF-67 for iodine could reach as high as 2000 mg·g -1.
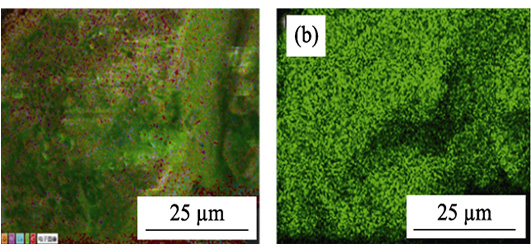
|
|
|
Adsorption of U(VI)-CO3/Ca-U(VI)-CO3 by Amidoxime-functionalized Hydrothermal Carbon
ZHANG Zhibin, ZHOU Runze, DONG Zhimin, CAO Xiaohong, LIU Yunhai
2020 Vol. 35 (3): 352–358
 Abstract
Abstract(
448 )
 HTML
HTML(
3)
 PDF
PDF(1049KB)(
723
)
Hydrothermal carbon adsorption materials possess the advantages of simple preparation process, mild synthesis conditions and easy surface modification. In this UO2 2+speciation as a function of CO3 2- concentration, soluble starch used as carbon source, acrylonitrile was grafted onto starch molecule through ring opening under the catalysis of cerium ammonium nitrate. Subsequently, amidoxime hydrothermal carbon spheres (AO-HTC) were successfully synthesized by hydrothermal reaction and hydroxylamine hydrochloride reduction. Meanwhile, static and dynamic adsorption experiments were performed to investigate the effects of solution pH, carbonate and calcium ion concentration on the adsorption performance of AO-HTC for uranium. And the dynamic adsorption process of AO-HTC for uranium was also studied by Yoon-Nelson and Thomas models. The results show that the adsorption capacity of AO-HTC, the volume of penetration point as well as saturation point in the penetration curve also decreases gradually with the increase of pH, carbonate concentration and calcium concentration. The maximum adsorption capacity (qo) and the required time (τ) of adsorbate through 50% of 5% AO-HTC column are several times higher than that of pure soil column. Therefore, the research highlights that AO-HTC would act as an excellent permeable-reactive barriers (PRB) medium and expected to remediate uranium-contaminated soil and groundwater.
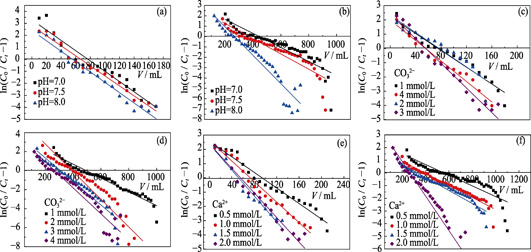
|
|
|
Construction of Novel Three Dimensionally Macroporous g-C3N4 for Efficient Adsorption/Photocatalytic Reduction of U(VI)
JIANG Li, GAO Huihui, CAO Ruya, ZHANG Shouwei, LI Jiaxing
2020 Vol. 35 (3): 359–366
 Abstract
Abstract(
723 )
 HTML
HTML(
27)
 PDF
PDF(2341KB)(
1022
)
Reduction of soluble U(VI) to insoluble U(IV) oxide is an effective approach to control uranium contamination. Three-dimensional (3D) macroporous g-C3N4 photocatalyst with interconnected porous was prepared by thermal polymerization and template etching using self-assembly of SiO2 nanosphere as the template. The material was then applied to adsorption-photocatalytic reduction of U(VI). Characterization results showed that the 3D macroporous g-C3N4 photocatalyst presented a well-defined interconnected macroporous architecture and numerous nanopores existed on the well-defined macroporous skeleton. 3D macroporous g-C3N4 also had a significant increase in specific surface area which was beneficial to the absorption of visible light. Adsorption results showed that the maximum adsorption capacity of U(VI) on 3D macroporous g-C3N4 was ~30.5 mg/g, which was more than ~1.83 times higher than that of bulk g-C3N4. The adsorption isotherm matched well with the Langumuir equation. Photocatalytic reduction experiments showed that the 3D macroporous g-C3N4 had high photocatalytic activity and good stability with the reduction rate constant of 0.0142 min -1, which was ~4.9 times higher than bulk g-C3N4 (~0.0024 min -1). As the sorption-photocatalytic performance of the sample is excellent, 3D macroporous g-C3N4 is a high efficient visible-light-responsive photocatalyst for the removal of U(VI) from radioactive wastewater.
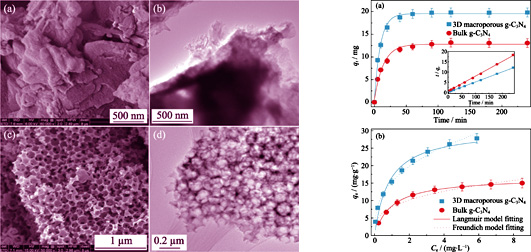
|
|
|
Layered Metal Organic Framework for Effective Removal of 137Cs from Aqueous Solution
LI Guodong, JI Guoxun, SUN Xinli, DU Wei, LIU Wei, WANG Shuao
2020 Vol. 35 (3): 367–372
 Abstract
Abstract(
681 )
 HTML
HTML(
15)
 PDF
PDF(1648KB)(
783
)
137Cs is one of the most intractable β-emitters which is commonly generated from nuclear weapons test and nuclear power station. Due to the nature of high solubility and mobility, the effective sequestration of 137Cs + from radioactive waste solution is considered as a long-term challenge. In this work, a two-dimensional layered anion framework material (SZ-6) was synthesized through conventional solvothermal reaction and the Cs + removal properties were systematically investigated. Single Crystal X-ray Diffraction (SCXRD) analysis revealed that SZ-6 adopts layer packed structure with large tetramethylammonium cations loaded between the layers which is greatly beneficial to cation exchange process. Powder X-ray Diffraction (XRD) and Scanning Electron Microscope (SEM) confirmed the material with high purity and excellent hydrolytic stability. Batch experiments were used to investigate the adsorption behavior towards Cs + in aqueous solutions. The adsorption kinetics of SZ-6 could be achieved within 5 min, which is currently one of the fastest sorbents for the removal of Cs +. Meanwhile, SZ-6 exhibits superior decontamination capability over a wide pH range from 4 to 12. Furthermore, it possesses marked selectivity in the presence of large excess of Na +, K +, Ca 2+ competing cations.
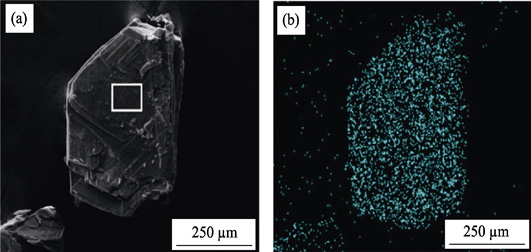
|
|
|
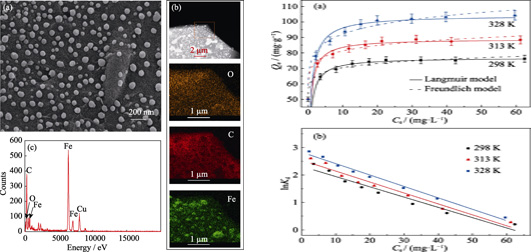
Carbothermic Synthesis of Carbon-supported Zero-valent Iron Material for Removal of U(Ⅵ) from Aqueous Solution
WANG Jiaqi, PANG Hongwei, TANG Hao, YU Shujun, ZHU Hongtao, WANG Xiangxue
2020 Vol. 35 (3): 373–380
 Abstract
Abstract(
880 )
 HTML
HTML(
25)
 PDF
PDF(3595KB)(
1046
)
With the development of nuclear power, radioactive pollutants discharge into the environment and then contaminate soil and water resources. Nanoscale zero-valent iron (nZVI) materials are widely used in water remediation due to their strong reducibility and high removal efficiency. A carbon-based zero-valent iron material (Fe-CB) was prepared in this work. Fe-CB was fabricated using sodium alginate (SA) as a carbon source via one-step carbothermic method and then applied to eliminate U(Ⅵ) from aqueous solution. Its mechanism and adsorption properties of Fe-CB and U(VI) were studied by spectroscopic analyses and macroscopic experiments. The results illustrated that Fe-CB possessed of ample functional groups (such as -OH and -COOH) and high BET surface area, which made up for the dispersibility and low removal efficiency of nanoscale zero-valent iron (nZVI). The removal of U(VI) by Fe-CB achieved equilibrium in 3 h and the maximum sorption capacity was 77.3 mg·g -1 at 298 K. XPS analyses indicated that the U(Ⅵ) removal by Fe-CB was a synergistic effect of reductive adsorptive processes. Adsorption process resulted from surface complexation and the reduction process was dominated by U(VI) reduction to U(IV) by nZVI. The results show that Fe-CB can be used as an inexpensive and highly efficient pollutant scavenger, which has great potential for environment pollution management.
|
|
|
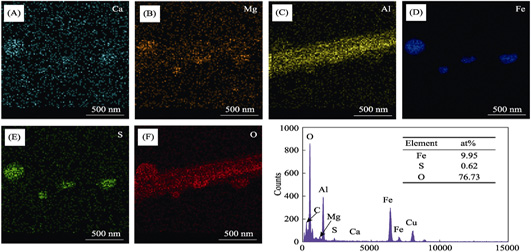
Ternary Layered Double Hydroxide Supported Sulfide NZVI: Efficient U(VI) Elimination and Mechanism
PANG Hongwei, TANG Hao, WANG Jiaqi, WANG Xiangxue, YU Shujun
2020 Vol. 35 (3): 381–389
 Abstract
Abstract(
682 )
 HTML
HTML(
22)
 PDF
PDF(2079KB)(
843
)
Nanoscale zero-valent iron (NZVI) has been widely applied to eliminate radionuclide U(VI). However, poor stability and low efficiency restrict the employment of pure NZVI. In this study, surface passivation and dispersion technology were employed together. Ca-Mg-Al layered double hydroxide supported sulfide NZVI (CMAL-SNZVI) was synthesized and applied for U(VI) elimination. Macroscopic and microscopic investigations demonstrate the outstanding physicochemical properties, high reactivity and excellent performance for U(VI) removal. The reaction process can be achieved equilibrium within 2 h and the maximum elimination capacity reaches 175.7 mg·g -1. The removal mechanism of U(VI) on CMAL-SNZVI is the synergistic effect between adsorption and reduction, through which U(VI) can be adsorbed by CMAL base and the SNZVI surface via inner-sphere surface complexation, U(VI) can be reduced into less toxic and insoluble U(IV) by Fe 0 inner core. Overall, the synthetization of CMAL-SNZVI can lead a new direction of NZVI modification. In the meantime, the outstanding performance of U(VI) removal indicate the potential of CMAL-SNZVI as excellent material for environment remediation.
|
|
|
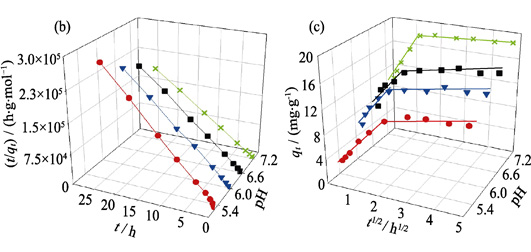
Sorption Behaviors and Mechanisms of Eu(III) on Rice Straw-derived Biochar
DONG Lijia, WU Siying, LI Shengbo, WEI Zuofu, YANG Guo, HU Baowei
2020 Vol. 35 (3): 390–398
 Abstract
Abstract(
544 )
 HTML
HTML(
22)
 PDF
PDF(1005KB)(
757
)
Biochar, derived from agricultural residuals, is extensively applied to remove detrimental heavy metals from wastewater, which has dual significance for the environment protection. Herein, the sorption behavior and interaction mechanism of Eu(III) on rice straw-derived biochar was investigated by batch and spectroscopic technologies. The solution pH significantly affects the percentage of the sorption, but has little effect on the contact time. Humic substances (HA/FA) significantly enhance Eu(III) sorption with solution pH<7.0, but inhibit the sorption with solution pH>7.0. The sorption mechanism involves co-precipitation or inner-sphere surface complexation. And the chemical sorption rate is restricted by intra-particle diffusion process. Besides, the Freundlich model simulates isotherms best and the maximum sorption amount is up to 40.717 mg/kg, which correlates with the stratified structure and abundant functional groups of biochar. The thermodynamic parameters suggest that the sorption of Eu(III) on biochar is a spontaneous and endothermic process. Therefore, these results are valuable to assess the potential application values of rice straw-derived biochar for the removal of Eu(III) in water systems.
|
|