
Journal of Inorganic Materials ›› 2022, Vol. 37 ›› Issue (10): 1141-1148.DOI: 10.15541/jim20220033
• RESEARCH LETTER • Previous Articles
WU Jing1( ), YU Libing1, LIU Shuaishuai1, HUANG Qiuyan1, JIANG Shanshan1, ANTON Matveev2, WANG Lianli3, SONG Erhong4(
), YU Libing1, LIU Shuaishuai1, HUANG Qiuyan1, JIANG Shanshan1, ANTON Matveev2, WANG Lianli3, SONG Erhong4( ), XIAO Beibei1(
), XIAO Beibei1( )
)
Received:2022-01-20
Revised:2022-04-06
Published:2022-10-20
Online:2022-05-09
Contact:
XIAO Beibei, associate professor. E-mail: xiaobb11@mails.jlu.edu.cn;About author:WU Jing(1998-), female, Master candidate. E-mail: wjjust20@163.com
Supported by:CLC Number:
WU Jing, YU Libing, LIU Shuaishuai, HUANG Qiuyan, JIANG Shanshan, ANTON Matveev, WANG Lianli, SONG Erhong, XIAO Beibei. NiN4/Cr Embedded Graphene for Electrochemical Nitrogen Fixation[J]. Journal of Inorganic Materials, 2022, 37(10): 1141-1148.
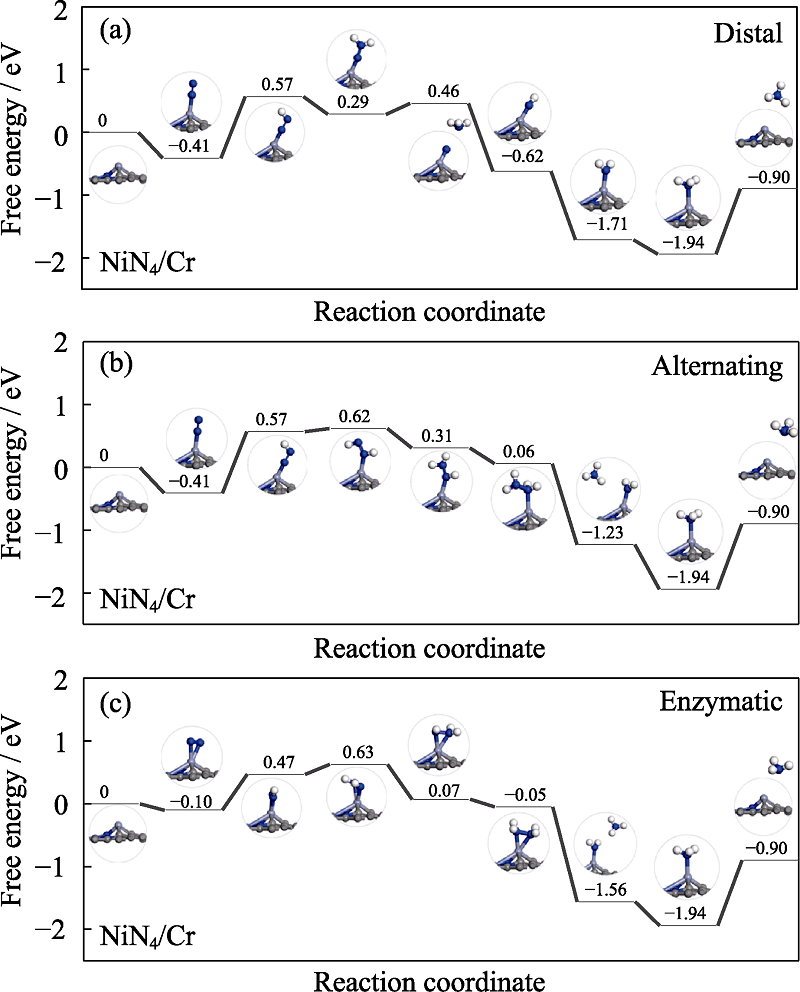
Fig. 4 Free energy diagrams and the corresponding configuration of the NRR intermediates on NiN4/Cr NRR mechanisms are (a) distal, (b) alternating and (c) enzymatic
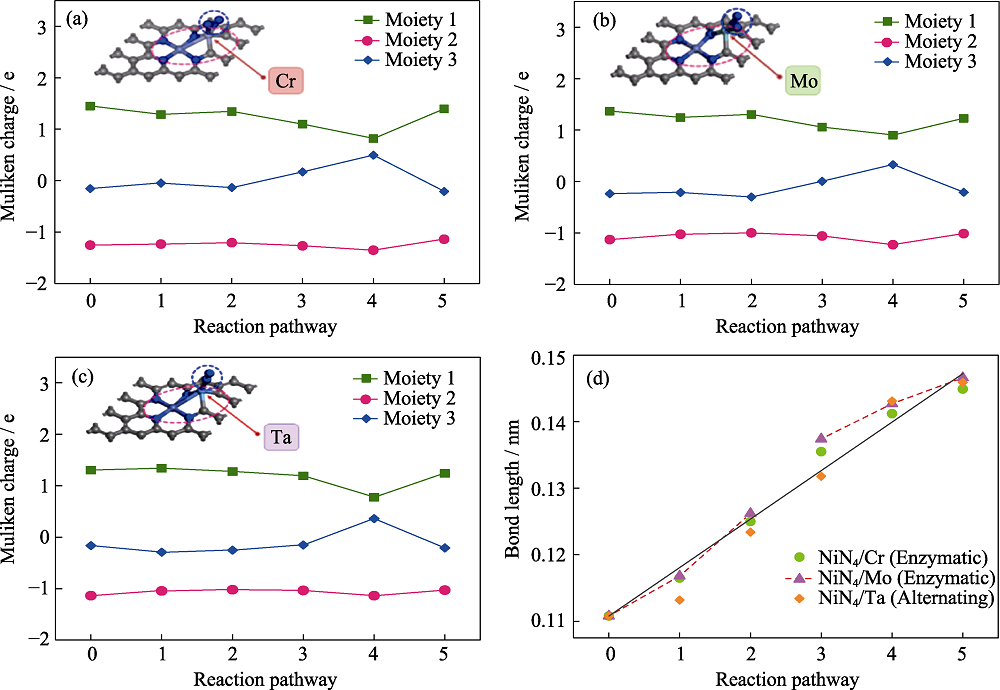
Fig. 5 (a-c) Charge variation of the three moieties along the optimal pathway and (d) N-N bond length change in NRR along preferred pathway Moieties 1, 2, 3 represent the graphene substrate, active center, and NRR intermediates, respectively
| 3d | Sc | Ti | V | Cr | Mn | Fe | Co | Ni |
|---|---|---|---|---|---|---|---|---|
| Eads(TM2) N2 end-on | -0.22 | -0.36 | -0.62 | -0.72 | -1.02 | -1.07 | -0.89 | -0.59 |
| Eads(TM2) N2 side-on | 0.12 | -0.02 | -1.17 | -0.35 | -0.59 | -0.51 | -0.33 | -0.19 |
| Eads(TM2) H | 0.75 | 0.20 | -0.18 | -0.14 | -0.19 | -0.20 | -0.22 | -0.38 |
| 4d | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd |
| Eads(TM2) N2 end-on | -0.14 | -0.22 | -1.05 | -0.70 | -0.73 | -0.99 | -0.73 | -1.30 |
| Eads(TM2) N2 side-on | -0.13 | 0.11 | -0.42 | -0.43 | -0.47 | -0.44 | -0.25 | -0.96 |
| Eads(TM2) H | 0.78 | 0.25 | -0.87 | -0.38 | 0.51 | -0.11 | -0.33 | -1.06 |
| 5d | Lu | Hf | Ta | W | Re | Os | Ir | Pt |
| Eads(TM2) N2 end-on | -0.21 | -0.35 | -0.60 | -1.57 | -1.23 | -1.30 | -1.08 | -0.52 |
| Eads(TM2) N2 side-on | 0.07 | 0.02 | -0.32 | -1.48 | -0.88 | -0.68 | -0.44 | -0.23 |
| Eads(TM2) H | 0.65 | -0.01 | -1.33 | -0.92 | -0.88 | -0.81 | -0.87 | -0.99 |
Table S1 Adsorption energies Eads on Mn1N4/TM2 (Eads in eV)
| 3d | Sc | Ti | V | Cr | Mn | Fe | Co | Ni |
|---|---|---|---|---|---|---|---|---|
| Eads(TM2) N2 end-on | -0.22 | -0.36 | -0.62 | -0.72 | -1.02 | -1.07 | -0.89 | -0.59 |
| Eads(TM2) N2 side-on | 0.12 | -0.02 | -1.17 | -0.35 | -0.59 | -0.51 | -0.33 | -0.19 |
| Eads(TM2) H | 0.75 | 0.20 | -0.18 | -0.14 | -0.19 | -0.20 | -0.22 | -0.38 |
| 4d | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd |
| Eads(TM2) N2 end-on | -0.14 | -0.22 | -1.05 | -0.70 | -0.73 | -0.99 | -0.73 | -1.30 |
| Eads(TM2) N2 side-on | -0.13 | 0.11 | -0.42 | -0.43 | -0.47 | -0.44 | -0.25 | -0.96 |
| Eads(TM2) H | 0.78 | 0.25 | -0.87 | -0.38 | 0.51 | -0.11 | -0.33 | -1.06 |
| 5d | Lu | Hf | Ta | W | Re | Os | Ir | Pt |
| Eads(TM2) N2 end-on | -0.21 | -0.35 | -0.60 | -1.57 | -1.23 | -1.30 | -1.08 | -0.52 |
| Eads(TM2) N2 side-on | 0.07 | 0.02 | -0.32 | -1.48 | -0.88 | -0.68 | -0.44 | -0.23 |
| Eads(TM2) H | 0.65 | -0.01 | -1.33 | -0.92 | -0.88 | -0.81 | -0.87 | -0.99 |
| 3d | Sc | Ti | V | Cr | Mn | Fe | Co | Ni |
|---|---|---|---|---|---|---|---|---|
| Eads(TM2) N2 end-on | -0.21 | -0.75 | -0.26 | -0.52 | -0.94 | -1.06 | -0.88 | -0.53 |
| Eads(TM2) N2 side-on | -0.21 | -0.37 | -0.35 | -0.41 | -0.59 | -0.54 | -0.25 | -0.56 |
| Eads(TM2) H | 0.93 | 0.33 | 0.27 | -0.02 | -0.14 | -0.25 | -0.11 | -0.37 |
| 4d | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd |
| Eads(TM2) N2 end-on | -0.14 | -0.22 | -0.20 | -0.62 | -0.88 | -0.96 | -0.76 | -0.49 |
| Eads(TM2) N2 side-on | 0.22 | -0.20 | -0.20 | 0.01 | -0.58 | -0.41 | -0.27 | 0.01 |
| Eads(TM2) H | 0.90 | 0.21 | 0.17 | -0.23 | -0.12 | -0.12 | -0.09 | -0.33 |
| 5d | Lu | Hf | Ta | W | Re | Os | Ir | Pt |
| Eads(TM2) N2 end-on | -0.20 | -0.31 | -0.64 | -0.91 | -1.15 | -1.27 | -1.09 | -0.26 |
| Eads(TM2) N2 side-on | -0.20 | 0.07 | -0.49 | -0.77 | -0.94 | -0.68 | -0.48 | 0.22 |
| Eads(TM2) H | 0.77 | 0.01 | -0.73 | -0.84 | -0.68 | -0.78 | -0.72 | -0.99 |
Table S2 Adsorption energies Eads on Fe1N4/TM2 (Eads in eV)
| 3d | Sc | Ti | V | Cr | Mn | Fe | Co | Ni |
|---|---|---|---|---|---|---|---|---|
| Eads(TM2) N2 end-on | -0.21 | -0.75 | -0.26 | -0.52 | -0.94 | -1.06 | -0.88 | -0.53 |
| Eads(TM2) N2 side-on | -0.21 | -0.37 | -0.35 | -0.41 | -0.59 | -0.54 | -0.25 | -0.56 |
| Eads(TM2) H | 0.93 | 0.33 | 0.27 | -0.02 | -0.14 | -0.25 | -0.11 | -0.37 |
| 4d | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd |
| Eads(TM2) N2 end-on | -0.14 | -0.22 | -0.20 | -0.62 | -0.88 | -0.96 | -0.76 | -0.49 |
| Eads(TM2) N2 side-on | 0.22 | -0.20 | -0.20 | 0.01 | -0.58 | -0.41 | -0.27 | 0.01 |
| Eads(TM2) H | 0.90 | 0.21 | 0.17 | -0.23 | -0.12 | -0.12 | -0.09 | -0.33 |
| 5d | Lu | Hf | Ta | W | Re | Os | Ir | Pt |
| Eads(TM2) N2 end-on | -0.20 | -0.31 | -0.64 | -0.91 | -1.15 | -1.27 | -1.09 | -0.26 |
| Eads(TM2) N2 side-on | -0.20 | 0.07 | -0.49 | -0.77 | -0.94 | -0.68 | -0.48 | 0.22 |
| Eads(TM2) H | 0.77 | 0.01 | -0.73 | -0.84 | -0.68 | -0.78 | -0.72 | -0.99 |
| 3d | Sc | Ti | V | Cr | Mn | Fe | Co | Ni |
|---|---|---|---|---|---|---|---|---|
| Eads(TM2) N2 end-on | -0.21 | -0.37 | -0.68 | -0.84 | -1.01 | -1.05 | -0.85 | -0.46 |
| Eads(TM2) N2 side-on | -0.20 | -0.37 | -0.29 | -0.51 | -0.64 | -0.53 | -0.26 | -0.56 |
| Eads(TM2) H | 1.02 | 0.37 | -0.09 | -0.08 | -0.36 | -0.13 | -0.07 | -0.28 |
| 4d | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd |
| Eads(TM2) N2 end-on | -0.12 | -0.19 | -0.44 | -0.61 | -0.82 | -0.93 | -0.75 | -0.48 |
| Eads(TM2) N2 side-on | -0.13 | -0.20 | -0.03 | -0.29 | -0.57 | -0.42 | -0.25 | -0.48 |
| Eads(TM2) H | 1.03 | 0.42 | -0.12 | -0.22 | -0.07 | -0.07 | -0.03 | -0.26 |
| 5d | Lu | Hf | Ta | W | Re | Os | Ir | Pt |
| Eads(TM2) N2 end-on | -0.20 | -0.29 | -0.62 | -0.86 | -1.08 | -1.23 | -1.07 | -0.49 |
| Eads(TM2) N2 side-on | -0.21 | -0.29 | -0.28 | -0.63 | -0.89 | -0.67 | -0.46 | -0.48 |
| Eads(TM2) H | 0.82 | 0.23 | -0.50 | -0.75 | -0.63 | -0.72 | -0.69 | -0.87 |
Table S3 Adsorption energies Eads on Co1N4/TM2 (Eads in eV)
| 3d | Sc | Ti | V | Cr | Mn | Fe | Co | Ni |
|---|---|---|---|---|---|---|---|---|
| Eads(TM2) N2 end-on | -0.21 | -0.37 | -0.68 | -0.84 | -1.01 | -1.05 | -0.85 | -0.46 |
| Eads(TM2) N2 side-on | -0.20 | -0.37 | -0.29 | -0.51 | -0.64 | -0.53 | -0.26 | -0.56 |
| Eads(TM2) H | 1.02 | 0.37 | -0.09 | -0.08 | -0.36 | -0.13 | -0.07 | -0.28 |
| 4d | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd |
| Eads(TM2) N2 end-on | -0.12 | -0.19 | -0.44 | -0.61 | -0.82 | -0.93 | -0.75 | -0.48 |
| Eads(TM2) N2 side-on | -0.13 | -0.20 | -0.03 | -0.29 | -0.57 | -0.42 | -0.25 | -0.48 |
| Eads(TM2) H | 1.03 | 0.42 | -0.12 | -0.22 | -0.07 | -0.07 | -0.03 | -0.26 |
| 5d | Lu | Hf | Ta | W | Re | Os | Ir | Pt |
| Eads(TM2) N2 end-on | -0.20 | -0.29 | -0.62 | -0.86 | -1.08 | -1.23 | -1.07 | -0.49 |
| Eads(TM2) N2 side-on | -0.21 | -0.29 | -0.28 | -0.63 | -0.89 | -0.67 | -0.46 | -0.48 |
| Eads(TM2) H | 0.82 | 0.23 | -0.50 | -0.75 | -0.63 | -0.72 | -0.69 | -0.87 |
| 3d | Sc | Ti | V | Cr | Mn | Fe | Co | Ni |
|---|---|---|---|---|---|---|---|---|
| Eads(TM2) N2 end-on | -0.21 | -0.41 | -0.72 | -0.91 | -1.04 | -1.07 | -0.79 | -0.58 |
| Eads(TM2) N2 side-on | -0.19 | 0.02 | -0.41 | -0.63 | -0.66 | -0.50 | / | -0.58 |
| Eads(TM2) H | 0.97 | 0.19 | -0.14 | -0.40 | -0.23 | -0.22 | -0.18 | -0.27 |
| 4d | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd |
| Eads(TM2) N2 end-on | -0.12 | -0.24 | -0.51 | -0.70 | -0.91 | -0.98 | -0.73 | -0.48 |
| Eads(TM2) N2 side-on | -0.13 | -0.20 | -0.23 | -0.63 | -0.61 | -0.44 | -0.21 | -0.48 |
| Eads(TM2) H | 0.97 | 0.15 | -0.33 | -0.22 | -0.12 | -0.13 | -0.16 | -0.25 |
| 5d | Lu | Hf | Ta | W | Re | Os | Ir | Pt |
| Eads(TM2) N2 end-on | -0.20 | -0.33 | -0.74 | -0.98 | -1.17 | -1.30 | -1.06 | -0.65 |
| Eads(TM2) N2 side-on | -0.20 | 0.06 | -0.49 | -0.94 | -0.95 | -0.68 | -0.41 | -0.65 |
| Eads(TM2) H | 0.85 | 0.02 | -0.71 | -0.66 | -0.69 | -0.82 | -0.81 | -0.93 |
Table S4 Adsorption energies Eads on Ni1N4/TM2 (Eads in eV)
| 3d | Sc | Ti | V | Cr | Mn | Fe | Co | Ni |
|---|---|---|---|---|---|---|---|---|
| Eads(TM2) N2 end-on | -0.21 | -0.41 | -0.72 | -0.91 | -1.04 | -1.07 | -0.79 | -0.58 |
| Eads(TM2) N2 side-on | -0.19 | 0.02 | -0.41 | -0.63 | -0.66 | -0.50 | / | -0.58 |
| Eads(TM2) H | 0.97 | 0.19 | -0.14 | -0.40 | -0.23 | -0.22 | -0.18 | -0.27 |
| 4d | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd |
| Eads(TM2) N2 end-on | -0.12 | -0.24 | -0.51 | -0.70 | -0.91 | -0.98 | -0.73 | -0.48 |
| Eads(TM2) N2 side-on | -0.13 | -0.20 | -0.23 | -0.63 | -0.61 | -0.44 | -0.21 | -0.48 |
| Eads(TM2) H | 0.97 | 0.15 | -0.33 | -0.22 | -0.12 | -0.13 | -0.16 | -0.25 |
| 5d | Lu | Hf | Ta | W | Re | Os | Ir | Pt |
| Eads(TM2) N2 end-on | -0.20 | -0.33 | -0.74 | -0.98 | -1.17 | -1.30 | -1.06 | -0.65 |
| Eads(TM2) N2 side-on | -0.20 | 0.06 | -0.49 | -0.94 | -0.95 | -0.68 | -0.41 | -0.65 |
| Eads(TM2) H | 0.85 | 0.02 | -0.71 | -0.66 | -0.69 | -0.82 | -0.81 | -0.93 |
| System | Mechanisms | N2 adsorption | R1 | R2 | R3 | R4 | R5 | R6 | NH3 desorption |
|---|---|---|---|---|---|---|---|---|---|
| NiN4/Cr | Distal | -0.41 | 0.98 | -0.28 | 0.17 | -1.08 | -1.09 | -0.23 | 1.04 |
| Alternating | -0.41 | 0.98 | 0.05 | -0.31 | -0.25 | -1.29 | -0.71 | 1.04 | |
| Enzymatic | -0.10 | 0.57 | 0.16 | -0.56 | -0.12 | -1.51 | -0.38 | 1.04 | |
| NiN4/Mo | Distal | -0.27 | 0.92 | -0.08 | -0.22 | -1.14 | -0.71 | -0.20 | 1.04 |
| Alternating | -0.27 | 0.92 | 0.16 | -0.56 | 0.06 | -1.52 | -0.49 | 1.04 | |
| Enzymatic | -0.11 | 0.60 | 0.18 | -0.89 | 0.50 | -1.54 | -0.44 | 1.04 | |
| NiN4/Ta | Distal | -0.18 | 0.69 | -0.37 | -0.06 | -1.22 | -1.02 | 0.22 | 1.04 |
| Alternating | -0.18 | 0.69 | 0.05 | -0.88 | 0.11 | -1.78 | 0.05 | 1.04 | |
| Enzymatic | 0.04 | 0.11 | -0.23 | -0.70 | 0.58 | -1.70 | -0.04 | 1.04 |
Table S5 Free energy change ΔG (ΔG in eV), Ri stands for the ith protonation step
| System | Mechanisms | N2 adsorption | R1 | R2 | R3 | R4 | R5 | R6 | NH3 desorption |
|---|---|---|---|---|---|---|---|---|---|
| NiN4/Cr | Distal | -0.41 | 0.98 | -0.28 | 0.17 | -1.08 | -1.09 | -0.23 | 1.04 |
| Alternating | -0.41 | 0.98 | 0.05 | -0.31 | -0.25 | -1.29 | -0.71 | 1.04 | |
| Enzymatic | -0.10 | 0.57 | 0.16 | -0.56 | -0.12 | -1.51 | -0.38 | 1.04 | |
| NiN4/Mo | Distal | -0.27 | 0.92 | -0.08 | -0.22 | -1.14 | -0.71 | -0.20 | 1.04 |
| Alternating | -0.27 | 0.92 | 0.16 | -0.56 | 0.06 | -1.52 | -0.49 | 1.04 | |
| Enzymatic | -0.11 | 0.60 | 0.18 | -0.89 | 0.50 | -1.54 | -0.44 | 1.04 | |
| NiN4/Ta | Distal | -0.18 | 0.69 | -0.37 | -0.06 | -1.22 | -1.02 | 0.22 | 1.04 |
| Alternating | -0.18 | 0.69 | 0.05 | -0.88 | 0.11 | -1.78 | 0.05 | 1.04 | |
| Enzymatic | 0.04 | 0.11 | -0.23 | -0.70 | 0.58 | -1.70 | -0.04 | 1.04 |
| Distal | Alternating | Enzymatic | ||||
|---|---|---|---|---|---|---|
| RDS | ΔGmax | RDS | ΔGmax | RDS | ΔGmax | |
| Cr | *N2+H→*NNH | 1.03 | *N2+H→*NNH | 1.03 | *N*N+H→*N*NH | 0.66 |
| NiN4/Cr | *N2+H→*NNH | 0.98 | *N2+H→*NNH | 0.98 | *N*N+H→*N*NH | 0.57 |
| Mo | *N2+H→*NNH | 1.27 | *N2+H→*NNH | 1.27 | *N*N+H→*N*NH | 0.43 |
| NiN4/Mo | *N2+H→*NNH | 0.92 | *N2+H→*NNH | 0.92 | *N*N+H→*N*NH | 0.60 |
| Ta | *NNH2+H→*N | 0.72 | *N2+H→*NNH | 0.66 | *NH*NH2+H→*NH2*NH2 | 0.49 |
| NiN4/Ta | *N2+H→*NNH | 0.69 | *N2+H→*NNH | 0.69 | *NH*NH2+H→*NH2*NH2 | 0.58 |
Table S6 Potential determining step and its free energy change ΔGmax(ΔGmax in eV)
| Distal | Alternating | Enzymatic | ||||
|---|---|---|---|---|---|---|
| RDS | ΔGmax | RDS | ΔGmax | RDS | ΔGmax | |
| Cr | *N2+H→*NNH | 1.03 | *N2+H→*NNH | 1.03 | *N*N+H→*N*NH | 0.66 |
| NiN4/Cr | *N2+H→*NNH | 0.98 | *N2+H→*NNH | 0.98 | *N*N+H→*N*NH | 0.57 |
| Mo | *N2+H→*NNH | 1.27 | *N2+H→*NNH | 1.27 | *N*N+H→*N*NH | 0.43 |
| NiN4/Mo | *N2+H→*NNH | 0.92 | *N2+H→*NNH | 0.92 | *N*N+H→*N*NH | 0.60 |
| Ta | *NNH2+H→*N | 0.72 | *N2+H→*NNH | 0.66 | *NH*NH2+H→*NH2*NH2 | 0.49 |
| NiN4/Ta | *N2+H→*NNH | 0.69 | *N2+H→*NNH | 0.69 | *NH*NH2+H→*NH2*NH2 | 0.58 |
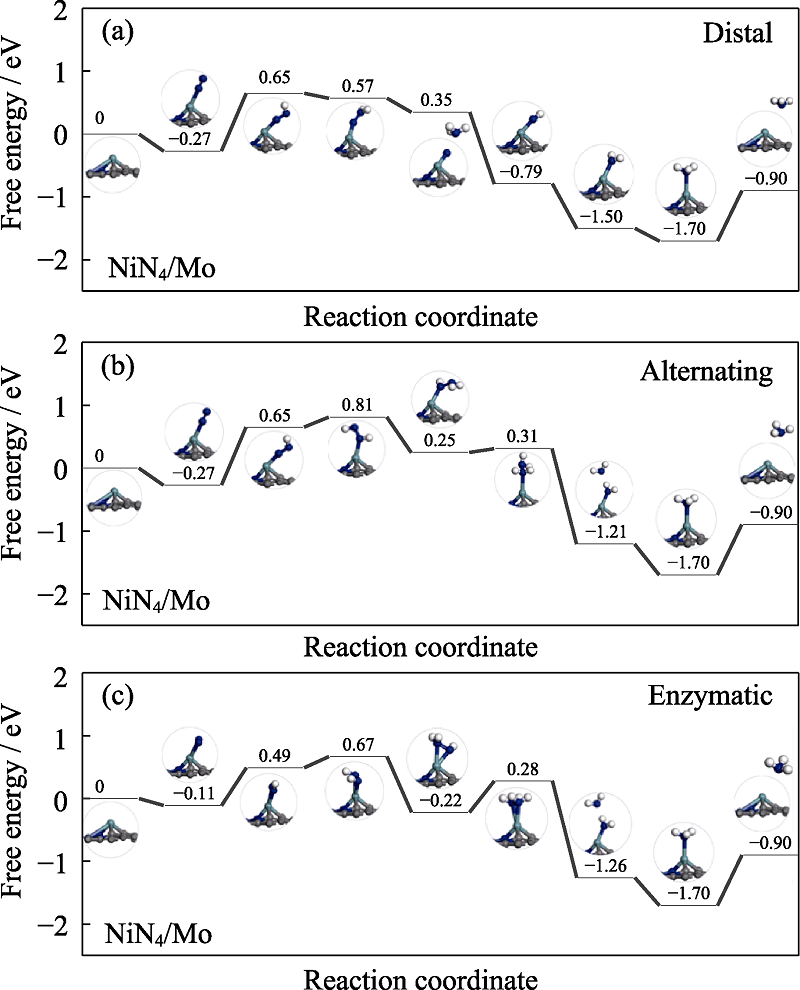
Fig. S2 Free energy diagrams and the corresponding configuration of the NRR intermediates on NiN4/Mo NRR mechanisms are (a) distal, (b) alternating, and (c) enzymatic, respectively
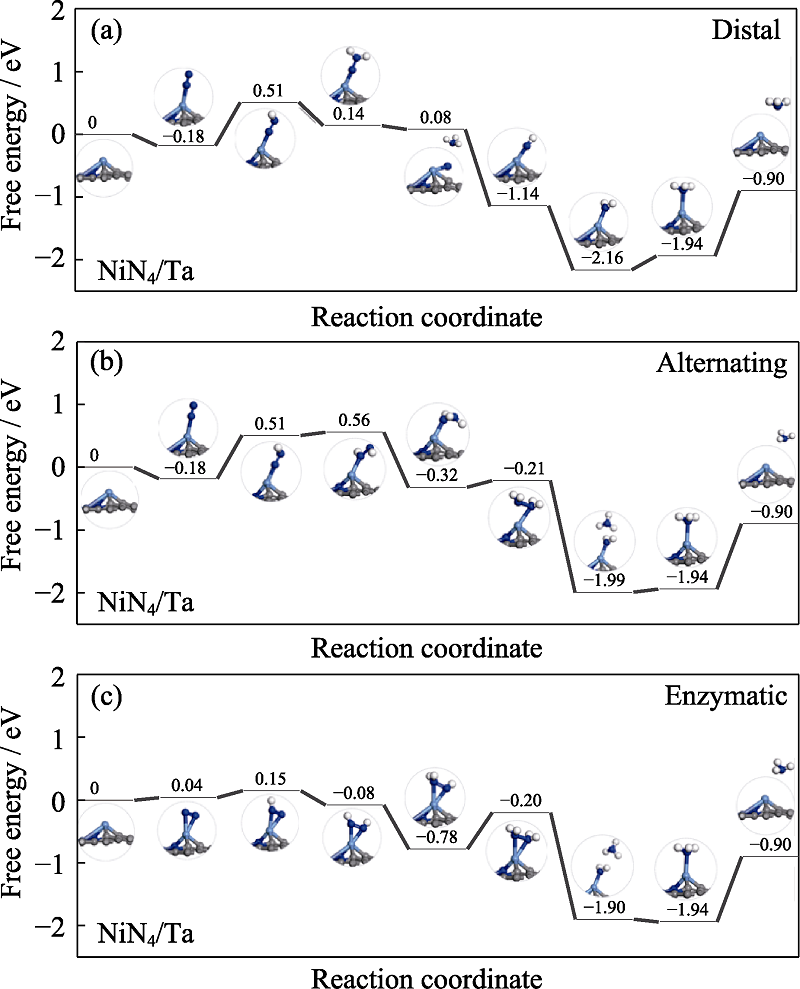
Fig. S3 Free energy diagrams and the corresponding configuration of the NRR intermediates on NiN4/Ta NRR mechanisms are (a) distal, (b) alternating, and (c) enzymatic, respectively
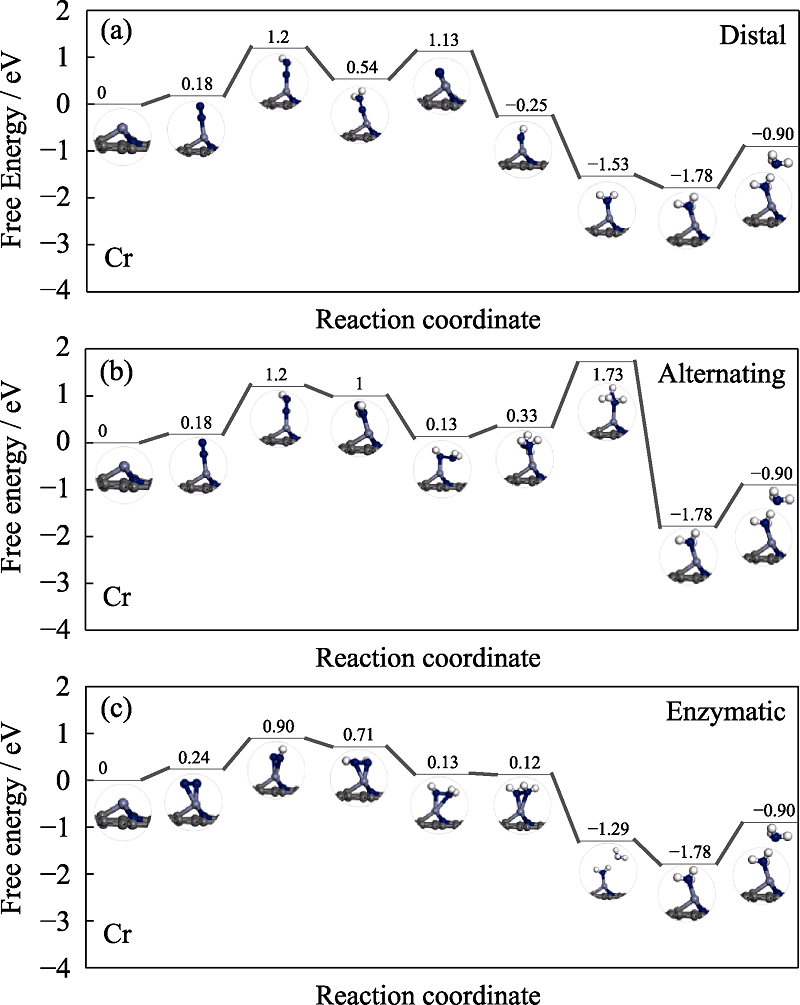
Fig. S4 Free energy diagrams and the corresponding configuration of the NRR intermediates on Cr embedded nitrogen functionalized graphene NRR mechanisms are (a) distal, (b) alternating, and (c) enzymatic, respectively
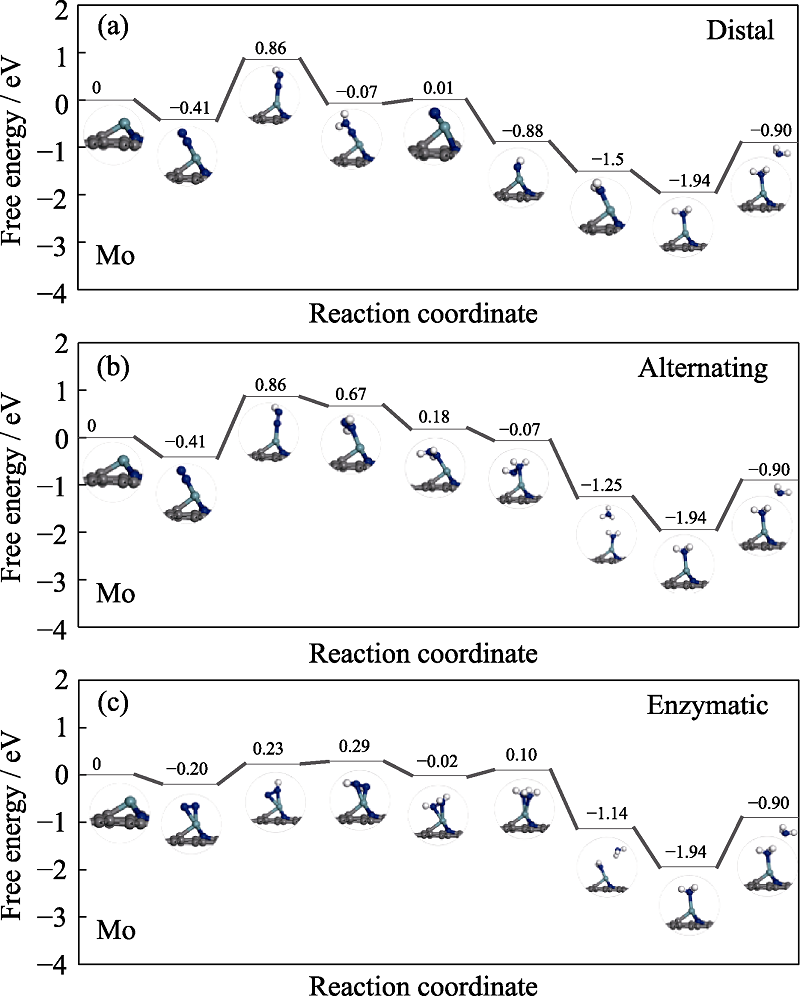
Fig. S5 Free energy diagrams and the corresponding configuration of the NRR intermediates on Mo embedded nitrogen functionalized graphene NRR mechanisms are (a) distal, (b) alternating, and (c) enzymatic, respectively
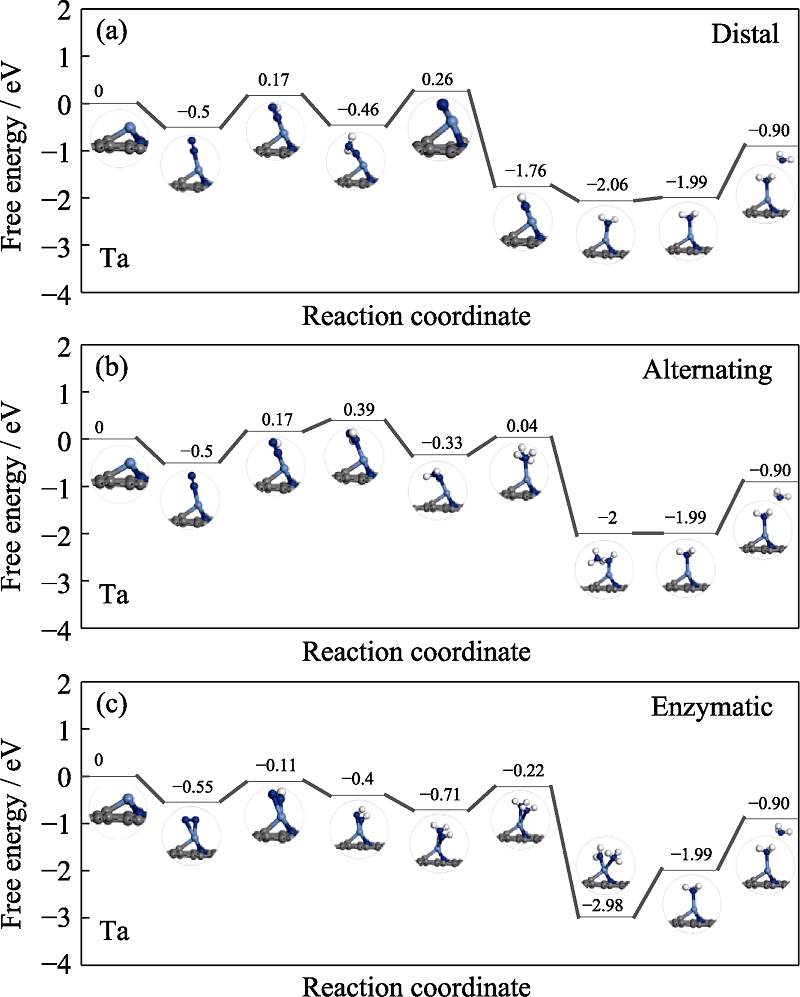
Fig. S6 Free energy diagrams and the corresponding configuration of the NRR intermediates on Ta embedded nitrogen functionalized graphene NRR mechanisms are (a) distal, (b) alternating, and (c) enzymatic, respectively
| [1] | WANG Y, JIA K, PAN Q, et al. Boron-doped TiO2 for efficient electrocatalytic N2 fixation to NH3 at ambient conditions. ACS Sustain. Chem. Engineer., 2018, 7(1): 117-122. |
| [2] |
LI H Y, YANG L, WANG Z X, et al. N-heterocyclic carbene as a promising metal-free electrocatalyst with high efficiency for nitrogen reduction to ammonia. J. Energy Chem., 2020, 46(7): 78-86.
DOI URL |
| [3] |
LI Q Y, HE L Z, SUN C H, et al. Computational study of MoN2 monolayer as electrochemical catalysts for nitrogen reduction. J. Phys. Chem. C, 2017, 121(49): 27563-27568.
DOI URL |
| [4] |
CHOI C, BACK S, KIM N Y, et al. Suppression of hydrogen evolution reaction in electrochemical N2 reduction using single- atom catalysts: a computational guideline. ACS Catal., 2018, 8(8): 7517-7525.
DOI URL |
| [5] |
ZHAO W H, ZHANG L F, LUO Q Q, et al. Single Mo1(Cr1) atom on nitrogen-doped graphene enables highly selective electroreduction of nitrogen into ammonia. ACS Catal., 2019, 9(4): 3419-3425.
DOI URL |
| [6] |
WANG S Y, SHI L, BAI X W, et al. Highly efficient photo-/ electrocatalytic reduction of nitrogen into ammonia by dual-metal sites. ACS Central Sci., 2020, 6(10): 1762-1771.
DOI URL |
| [7] |
MA B Y, PENG Y, Ma D W, et al. Boron-doped InSe monolayer as a promising electrocatalyst for nitrogen reduction into ammonia at ambient conditions. Appl. Surf. Sci., 2019, 495(30): 143463.
DOI URL |
| [8] |
LING C Y, NIU X H, LI Q, et al. Metal-free single atom catalyst for N2 fixation driven by visible light. J. Am. Chem. Soc., 2018, 140(43): 14161-14168.
DOI URL |
| [9] |
LIU X, WANG Z X, ZHAO J, et al. Two-dimensional π-conjugated osmium bis(dithiolene) complex (OsC4S4) as a promising electrocatalyst for ambient nitrogen reduction to ammonia. Appl. Surf. Sci., 2019, 487(1): 833-839.
DOI URL |
| [10] |
JI S, WANG Z X, ZHAO J X. A boron-interstitial doped C2N layer as a metal-free electrocatalyst for N2 fixation: a computational study. J. Mater. Chem. A, 2019, 7(5): 2392-2399.
DOI URL |
| [11] |
ZHANG X, CHEN A, ZHANG Z H, et al. Double-atom catalysts: transition metal dimer-anchored C2N monolayers as N2 fixation electrocatalysts. J. Mater. Chem. A, 2018, 6(38): 18599-18604.
DOI URL |
| [12] |
LI F F, CHEN L, LIU H M, et al. Enhanced N2-fixation by engineering the edges of two-dimensional transition-metal disulfides. J. Phys. Chem. C, 2019, 123(36): 22221-22227.
DOI URL |
| [13] |
QIN G Q, CUI Q Y, DU A J, et al. Transition metal diborides: a new type of high-performance electrocatalysts for nitrogen reduction. ChemCatChem, 2019, 11(11): 2624-2633.
DOI URL |
| [14] |
ZHU H R, HU Y L, WEI S H, et al. Single-metal atom anchored on boron monolayer (β12) as an electrocatalyst for nitrogen reduction into ammonia at ambient conditions: a first-principles study. J. Phys. Chem. C, 2019, 123(7): 4274-4281.
DOI URL |
| [15] |
WANG S M, ZHANG L, QIN Y, et al. Co, N-codoped graphene as efficient electrocatalyst for hydrogen evolution reaction: insight into the active centre. J. Power Sources, 2017, 363(30): 260-268.
DOI URL |
| [16] |
YANG Y L, LIU J D, WEI Z X, et al. Transition metal-dinitrogen complex embedded graphene for nitrogen reduction reaction. ChemCatChem, 2019, 11(12): 2821-2827.
DOI URL |
| [17] |
RIYAZ M, GOEL N. Single-atom catalysis using chromium embedded in divacant graphene for conversion of dinitrogen to ammonia. ChemPhysChem, 2019, 20(15): 1954-1959.
DOI PMID |
| [18] |
SUN C N, WANG Z L, LANG X Y, et al. Synergistic effect of active sites of double-atom catalysts for nitrogen reduction reaction. ChemSusChem, 2021, 14(20): 4593-4600.
DOI URL |
| [19] |
ZHENG X N, YAO Y, WANG Y, et al. Tuning the electronic structure of transition metals embedded in nitrogen-doped graphene for electrocatalytic nitrogen reduction: a first-principles study. Nanoscale, 2020, 12(17): 9696-9707.
DOI PMID |
| [20] | ZHOU Y, SONG E H, CHEN W, et al. Dual-metal interbonding as the chemical facilitator for single-atom dispersions. Adv. Mater., 2020, 32(46): e2003484. |
| [21] | DELLY B. An all-electron numerical method for solving the local density functional for polyatomic molecules. J. Chem. Phys., 1990, 92(1): 508-517. |
| [22] |
DELLY B. From molecules to solids with the DMol3 approach. J. Chem. Phys., 2000, 113(18): 7756-7764.
DOI URL |
| [23] |
OERDEW J P, BURKE K, ERNZERHOF M. Generalized gradient approximation made simple. Phys. Rev. Lett., 1996, 77(18): 3865-3868.
DOI PMID |
| [24] |
DELLY B. Hardness conserving semilocal pseudopotentials. Phys. Rev. B, 2002, 66(15): 155125.
DOI URL |
| [25] |
TODOROVA T, DELLY B. Wetting of paracetamol surfaces studied by DMol3-COSMO calculations. Mol. Simulat., 2008, 34(10/15): 1013-1017.
DOI URL |
| [26] |
WEI Z X, ZHANG Y F, WANG S Y, et al. Fe-doped phosphorene for the nitrogen reduction reaction. J. Mater. Chem. A, 6(28): 13790-13796.
DOI URL |
| [27] |
SHI L, LI Q, LING C Y, et al. Metal-free electrocatalyst for reducing nitrogen to ammonia using a Lewis acid pair. J. Mater. Chem. A, 2019, 7(9): 4865-4871.
DOI URL |
| [28] |
JIANG C H, ZHOU R Q, PENG Z H, et al. An atomically thin layer of Ru/MoS2 heterostructure: structural, electronic, and magnetic properties. Phys. Chem. Chem. Phys., 2016, 18(47): 32528-32533.
DOI URL |
| [29] |
YU X M, HAN P, WEI Z X, et al. Boron-doped graphene for electrocatalytic N2 reduction. Joule, 2018, 2(8): 1610-1622.
DOI URL |
| [30] |
NORSKOV J K, ROSSMEISL J, LOGADOTTIR A, et al. Origin of the overpotential for oxygen reduction at a fuel-cell cathode. J. Phys. Chem. B, 2004, 108(46): 17886-17892.
DOI URL |
| [31] |
YANG L, FENG S, ZHU W. Tuning nitrate electroreduction activity via an equilibrium adsorption strategy: a computational study. J Phys. Chem. Lett., 2022, 13(7): 1726-1733.
DOI URL |
| [32] |
LIM D H, WILCOX J. Mechanisms of the oxygen reduction reaction on defective graphene-supported Pt nanoparticles from first-principles. J. Phys. Chem. C, 2012, 116(5): 3653-3660.
DOI URL |
| [33] |
YIN J, FANG Q H, LI Y X, et al. Ni-C-N nanosheets as catalyst for hydrogen evolution reaction. J. Am. Chem. Soc., 2016, 138(44): 14546-14549.
PMID |
| [34] |
LIANG H W, BRULLER S, DONG R H, et al. Molecular metal-Nx centres in porous carbon for electrocatalytic hydrogen evolution. Nat. Commun., 2015, 6(1): 7992.
DOI URL |
| [35] | LING C Y, OUYANG Y X, LI Q, et al. A general two-step strategy-based high-throughput screening of single atom catalysts for nitrogen fixation. Small Methods, 2019, 3(9): 1-8. |
| [36] |
QI J M, GAO L Y, WEI F F, et al. Design of a high-performance electrocatalyst for N2 conversion to NH3 by trapping single metal atoms on stepped CeO2. ACS Appl. Mater. Interfaces, 2019, 11(50): 47525-47534.
DOI URL |
| [37] |
SKULASON E, BLIFAARD T, GUDMUNDSDOTTIR S. A theoretical evaluation of possible transition metal electro- catalysts for N2 reduction. Phys. Chem. Chem. Phys., 2012, 14(3): 1235-1245.
DOI URL |
| [38] |
CHOI W I, WOOD B C, SCHWEGLER E, et al. Combinatorial search for high-activity hydrogen catalysts based on transition- metal-embedded graphitic carbons. Adv. Energy Mater., 2015, 5(23): 1501423.
DOI URL |
| [39] |
LIU C W, LI Q Y, ZHANG J, et al. Conversion of dinitrogen to ammonia on Ru atoms supported on boron sheets: a DFT study. J. Mater. Chem. A, 2019, 7(9): 4771-4776.
DOI URL |
| [40] |
LI X F, LI Q K, CHENG J, et al. Conversion of dinitrogen to ammonia by FeN3-embedded graphene. J. Am. Chem. Soc., 2016, 138(28): 8706-8709.
DOI URL |
| [41] |
QIU W B, XIE X Y, QIU J D, et al. High-performance artificial nitrogen fixation at ambient conditions using a metal-free electrocatalyst. Nat. Commun., 2018, 9(1): 3485.
DOI PMID |
| [42] |
GUO Y, GU J X, ZHANG R, et al. Molecular crowding effect in aqueous electrolytes to suppress hydrogen reduction reaction and enhance electrochemical nitrogen reduction. Adv. Energy Mater., 2021, 11(36): 2101699.
DOI URL |
| [43] |
GUO Y X, YAO Z Y, TIMMER B J J, et al. Boosting nitrogen reduction reaction by bio-inspired FeMoS containing hybrid electrocatalyst over a wide pH range. Nano Energy, 2019, 62: 282-288.
DOI URL |
| [44] |
WANG X, FENG Z, XIAO B, et al. Polyoxometalate-based metal-organic framework-derived bimetallic hybrid materials for upgraded electrochemical reduction of nitrogen. Green Chem., 2020, 22(18): 6157-6169.
DOI URL |
| [45] |
ZHAO J X, CHEN Z F. Single Mo atom supported on defective boron nitride monolayer as an efficient electrocatalyst for nitrogen fixation: a computational study. J. Am. Chem. Soc., 2017, 139(36): 12480-12487.
DOI PMID |
| [46] |
LING C Y, BAI X W, OUYANG Y X, et al. Single molybdenum atom anchored on N-doped carbon as a promising electrocatalyst for nitrogen reduction into ammonia at ambient conditions. J. Phys. Chem. C, 2018, 122(29): 16842-16847.
DOI URL |
| [47] |
WU Y B, HE C, ZHANG W X. “Capture-backdonation-recapture” mechanism for promoting N2 reduction by heteronuclear metal- free double-atom catalysts. J Am. Chem. Soc. 2022, 144(21): 9344-9353.
DOI URL |
| [1] | CHEN Saisai, PANG Yali, WANG Jiaona, GONG Yan, WANG Rui, LUAN Xiaowan, LI Xin. Preparation and Properties of Green-yellow Reversible Electro-thermochromic Fabric [J]. Journal of Inorganic Materials, 2022, 37(9): 954-960. |
| [2] | HU Yue, AN Lin, HAN Xin, HOU Chengyi, WANG Hongzhi, LI Yaogang, ZHANG Qinghong. RhO2 Modified BiVO4 Thin Film Photoanodes: Preparation and Photoelectrocatalytic Water Splitting Performance [J]. Journal of Inorganic Materials, 2022, 37(8): 873-882. |
| [3] | SUN Lian, GU Quanchao, YANG Yaping, WANG Honglei, YU Jinshan, ZHOU Xingui. Two-dimensional Transition Metal Dichalcogenides for Electrocatalytic Oxygen Reduction Reaction [J]. Journal of Inorganic Materials, 2022, 37(7): 697-709. |
| [4] | SUN Ming, SHAO Puzhen, SUN Kai, HUANG Jianhua, ZHANG Qiang, XIU Ziyang, XIAO Haiying, WU Gaohui. First-principles Study on Interface of Reduced Graphene Oxide Reinforced Aluminum Matrix Composites [J]. Journal of Inorganic Materials, 2022, 37(6): 651-659. |
| [5] | WANG Peng, JIN Zunlong, CHEN Ningguang, LIU Yonghao. Theoretical Investigation of Mo Doped α-MnO2 Electrocatalytic Oxygen Evolution Reaction [J]. Journal of Inorganic Materials, 2022, 37(5): 541-546. |
| [6] | WANG Hongli, WANG Nan, WANG Liying, SONG Erhong, ZHAO Zhankui. Hydrogen Generation from Formic Acid Boosted by Functionalized Graphene Supported AuPd Nanocatalysts [J]. Journal of Inorganic Materials, 2022, 37(5): 547-553. |
| [7] | AN Lin, WU Hao, HAN Xin, LI Yaogang, WANG Hongzhi, ZHANG Qinghong. Non-precious Metals Co5.47N/Nitrogen-doped rGO Co-catalyst Enhanced Photocatalytic Hydrogen Evolution Performance of TiO2 [J]. Journal of Inorganic Materials, 2022, 37(5): 534-540. |
| [8] | DONG Shurui, ZHAO Di, ZHAO Jing, JIN Wanqin. Effect of Ionized Amino Acid on the Water-selective Permeation through Graphene Oxide Membrane in Pervaporation Process [J]. Journal of Inorganic Materials, 2022, 37(4): 387-394. |
| [9] | JIANG Lili, XU Shuaishuai, XIA Baokai, CHEN Sheng, ZHU Junwu. Defect Engineering of Graphene Hybrid Catalysts for Oxygen Reduction Reactions [J]. Journal of Inorganic Materials, 2022, 37(2): 215-222. |
| [10] | FU Yongsheng, BI Min, LI Chun, SUN Jingwen, WANG Xin, ZHU Junwu. Research Progress on Non-noble Metal/Nitrogen-doped Carbon Composite Materials in Electrocatalytic Oxygen Evolution Reaction [J]. Journal of Inorganic Materials, 2022, 37(2): 163-172. |
| [11] | WEI Hailang, CAO Xueqiang, DENG Longhui, JIANG Jianing. Thermodynamic Properties and Thermal Cycling Lifetimes of LaMeAl11O19/YSZ Thermal Barrier Coatings [J]. Journal of Inorganic Materials, 2022, 37(12): 1259-1266. |
| [12] | LI Tie, LI Yue, WANG Yingyi, ZHANG Ting. Preparation and Catalytic Properties of Graphene-Bismuth Ferrite Nanocrystal Nanocomposite [J]. Journal of Inorganic Materials, 2021, 36(7): 725-732. |
| [13] | SU Li, YANG Jianping, LAN Yue, WANG Lianjun, JIANG Wan. Interface Design of Iron Nanoparticles for Environmental Remediation [J]. Journal of Inorganic Materials, 2021, 36(6): 561-569. |
| [14] | XIANG Hui, QUAN Hui, HU Yiyuan, ZHAO Weiqian, XU Bo, YIN Jiang. Piezoelectricity of Graphene-like Monolayer ZnO and GaN [J]. Journal of Inorganic Materials, 2021, 36(5): 492-496. |
| [15] | ZHOU Yuzhu, ZHANG Youkui, SONG Li. Noble Metal Phosphide Electrocatalysts and Their Synchrotron-based X-ray Absorption Spectroscopy [J]. Journal of Inorganic Materials, 2021, 36(3): 225-244. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||