
Journal of Inorganic Materials ›› 2022, Vol. 37 ›› Issue (10): 1058-1064.DOI: 10.15541/jim20220093
• RESEARCH ARTICLE • Previous Articles Next Articles
GAN Hongyu1( ), FENG Yan1, YANG Dehong1, TIAN Yubin1, LI Yang1, XING Tao2, LI Zhi2,3, ZHAO Xuebo1, DAI Pengcheng1(
), FENG Yan1, YANG Dehong1, TIAN Yubin1, LI Yang1, XING Tao2, LI Zhi2,3, ZHAO Xuebo1, DAI Pengcheng1( )
)
Received:2022-02-28
Revised:2022-03-26
Published:2022-10-20
Online:2022-04-07
Contact:
DAI Pengcheng, associate professor. E-mail: dpcapple@upc.edu.cnAbout author:GAN Hongyu (1998-), male, Master candidate. E-mail: hongyugan@163.com
Supported by:CLC Number:
GAN Hongyu, FENG Yan, YANG Dehong, TIAN Yubin, LI Yang, XING Tao, LI Zhi, ZHAO Xuebo, DAI Pengcheng. Heteroatom-doped Biochar for Direct Dehydrogenation of Propane to Propylene[J]. Journal of Inorganic Materials, 2022, 37(10): 1058-1064.
| Sample | C/% | N/% | O/% | B/% |
|---|---|---|---|---|
| B-BC | 82.6 | - | 16.57 | 0.83 |
| N-BC | 68.38 | 17.2 | 14.42 | - |
| BN-BC | 66.13 | 18.65 | 14.09 | 1.13 |
| BC | 83.76 | - | 16.24 | - |
Table 1 Component analyses of samples B-BC, N-BC, BN-BC, and BC (atom fraction)
| Sample | C/% | N/% | O/% | B/% |
|---|---|---|---|---|
| B-BC | 82.6 | - | 16.57 | 0.83 |
| N-BC | 68.38 | 17.2 | 14.42 | - |
| BN-BC | 66.13 | 18.65 | 14.09 | 1.13 |
| BC | 83.76 | - | 16.24 | - |
| Sample | SBET/(m2·g-1) | Vtotal/(cm3·g-1) | Pore diameter/nm |
|---|---|---|---|
| N-BC | 1303 | 0.582 | 0.79-2.10 |
| BN-BC | 1226 | 0.535 | 0.62-4.50 |
| B-BC | 765 | 0.281 | 0.73-3.50 |
| BC | 497 | 0.191 | 0.62-0.87 |
Table 2 Specific surface area, pore volume and average pore size of the samples
| Sample | SBET/(m2·g-1) | Vtotal/(cm3·g-1) | Pore diameter/nm |
|---|---|---|---|
| N-BC | 1303 | 0.582 | 0.79-2.10 |
| BN-BC | 1226 | 0.535 | 0.62-4.50 |
| B-BC | 765 | 0.281 | 0.73-3.50 |
| BC | 497 | 0.191 | 0.62-0.87 |
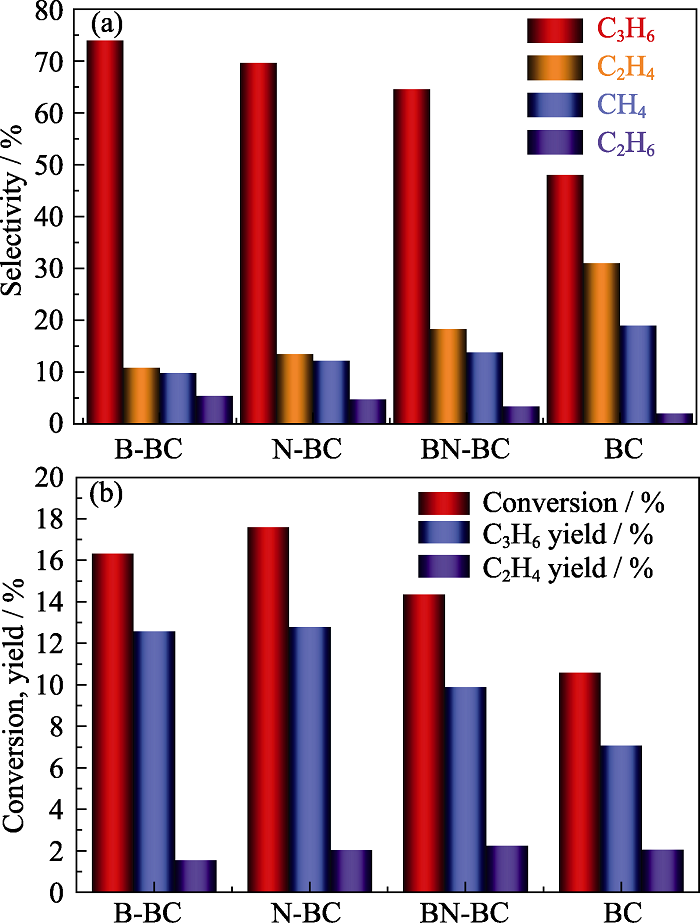
Fig. 6 Product distribution at the same conversion (20%) (a) and conversion and olefins yield of propane (b) over different carbon catalysts at reaction temperatures of 600 ℃ The reaction conditions: 0.5 g catalyst, He-to-propane ratio = 3, GHSV = 3840 mL·g-1·h-1 Colorful figures are available on website
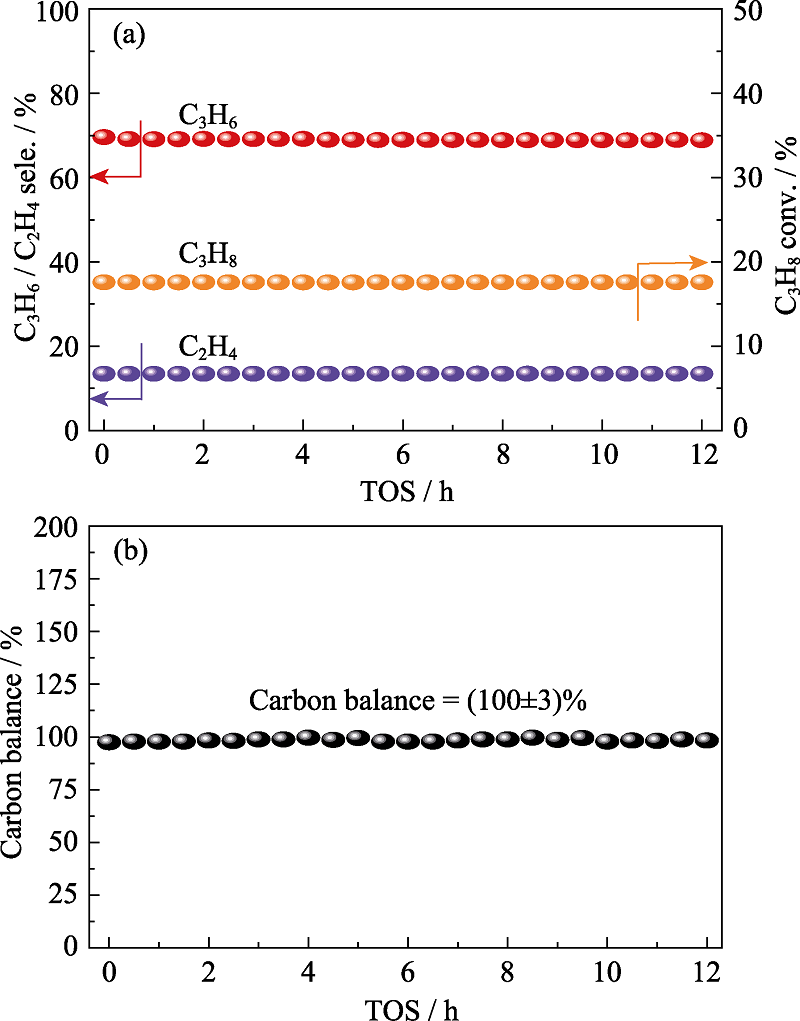
Fig. 8 Stability test (a) and carbon balance during the catalytic test (b) of N-BC for direct dehydrogenation (DDH) reaction over 12 h GHSV = 3840 mL·g-1·h-1; TOS: Time on stream
| Catalyst | T/℃ | X% (C3H8) | Y% (Olefins) | Ref. |
|---|---|---|---|---|
| N-BC | 600 | 17.6 | 14.8 | This work |
| BN-BC | 600 | 14.35 | 12.1 | This work |
| B-BC | 600 | 16.31 | 14.1 | This work |
| BC | 600 | 10.58 | 9.1 | This work |
| 5Cr2O3/SBA-15 | 580 | 18.0 | 14.8 | [ |
| PtSn/HZSM-5 | 590 | 22.9 | 11.2 | [ |
| CNTs | 600 | 9.0 | 7.9 | [ |
| GC | 600 | 6.5 | 6.1 | [ |
Table S1 Catalytic performance of some catalysts for direct dehydrogenation of propane
| Catalyst | T/℃ | X% (C3H8) | Y% (Olefins) | Ref. |
|---|---|---|---|---|
| N-BC | 600 | 17.6 | 14.8 | This work |
| BN-BC | 600 | 14.35 | 12.1 | This work |
| B-BC | 600 | 16.31 | 14.1 | This work |
| BC | 600 | 10.58 | 9.1 | This work |
| 5Cr2O3/SBA-15 | 580 | 18.0 | 14.8 | [ |
| PtSn/HZSM-5 | 590 | 22.9 | 11.2 | [ |
| CNTs | 600 | 9.0 | 7.9 | [ |
| GC | 600 | 6.5 | 6.1 | [ |
| [1] |
SATTLER J J H B, JAVIER RM, ELDVARDO S J, et al. Catalytic Dehydrogenation of light alkanes on Metals and metal oxides. Chemical Reviews, 2014, 114(20): 10613-10653.
DOI PMID |
| [2] |
CAVANI F, BALLARINI N, CERICOLA A. Oxidative dehydrogenation of ethane and propane: how far from commercial implementation? Catalysis Today, 2007, 127(1): 113-131.
DOI URL |
| [3] |
ZHAO Z, GE G, LI W, et al. Modulating the microstructure and surface chemistry of carbocatalysts for oxidative and direct dehydrogenation: a review. Chinese Journal of Catalysis, 2016, 37(5): 644-670.
DOI URL |
| [4] |
WECKHUYSEN B M, SCHOONHEYDT R A. Alkane dehydrogenation over supported chromium oxide catalysts. Catalysis Today, 1999, 51(2): 223-232.
DOI URL |
| [5] |
PHAM H N, SATTLER J J H B, WECKHUYSEN B M, et al. Role of Sn in the regeneration of Pt/γ-Al2O3 light alkane dehydrogenation catalysts. ACS Catalysis, 2016, 6(4): 2257-2264.
DOI URL |
| [6] |
MOTAGAMWALA A H, ALMALLAHI R, WORTMAN J, et al. Stable and selective catalysts for propane dehydrogenation operating at thermodynamic limit. Science, 2021, 373(6551): 217-222.
DOI PMID |
| [7] | HU Z P, ZHANG L F, WANG Z, et al. Bean dregs-derived hierarchical porous carbons as metal-free catalysts for efficient dehydrogenation of propane to propylene. Journal of Chemical Technology and Biotechnology, 2018, 93(12): 3410-3417. |
| [8] | PARAKNOWITSCH J P, THOMAS A. Doping carbons beyond nitrogen: an overview of advanced heteroatom doped carbons with boron, sulphur and phosphorus for energy applications. Energy & Environmental Science, 2013, 6(10): 2839-2855. |
| [9] |
SHENG J, YAN B, LU W D, et al. Oxidative dehydrogenation of light alkanes to olefins on metal-free catalysts. Chemical Society Reviews, 2021, 50(2): 1438-1468.
DOI PMID |
| [10] |
FRANK B, ZHANG J, BLUME R, et al. Heteroatoms increase the selectivity in oxidative dehydrogenation reactions on nanocarbons. Angewandte Chemie International Edition, 2009, 48(37): 6913-6917.
DOI URL |
| [11] | LIU L, DENG Q F, AGULA B, et al. Ordered mesoporous carbon catalyst for dehydrogenation of propane to propylene. Chemicial Communications, 2011, 47(29): 8334-8336. |
| [12] |
SONG Y, LIU G, YUAN Z Y. N-, P- and B-doped mesoporous carbons for direct dehydrogenation of propane. RSC Advances, 2016, 6(97): 94636-94642.
DOI URL |
| [13] |
SÁNCHEZ-MONEDERO M A, SÁNCHEZ-GARCíA M, ALBURQUERQUE J A, et al. Biochar reduces volatile organic compounds generated during chicken manure composting. Bioresource Technology, 2019, 288: 121584.
DOI URL |
| [14] | LIU W J, JIANG H, YU H Q. Emerging applications of biochar- based materials for energy storage and conversion. Energy & Environmental Science, 2019, 12(6): 1751-1779. |
| [15] |
DE JESÚS DÍAZ VELÁSQUEZ J, SUÁREZ L M C, FIGUEIREDO J L. Oxidative dehydrogenation of isobutane overactivated carbon catalysts. Applied Catalysis A: General, 2006, 311: 51-57.
DOI URL |
| [16] |
LI L, ZHONG Q, KIM N D, et al. Nitrogen-doped carbonized cotton for highly flexible supercapacitors. Carbon, 2016, 105: 260-267.
DOI URL |
| [17] |
KIM N D, KIM S J, KIM G P, et al. NH3-activated polyaniline for use as a high performance electrode material in supercapacitors. Electrochimica Acta, 2012, 78: 340-346.
DOI URL |
| [18] | DAI P, XUE Y, ZHANG S, et al. Paper-derived flexible 3D interconnected carbon microfiber networks with controllable pore sizes for supercapacitors. ACS Applied Materials & Interfaces, 2018, 10(43): 37046-37056. |
| [19] | CHHETRI M, MAITRA S, CHAKRABORTY H, et al. Superior performance of borocarbonitrides, BxCyNz, as stable, low-cost metal-free electrocatalysts for the hydrogen evolution reaction. Energy & Environmental Science, 2016, 9(1): 95-101. |
| [20] |
LI Y T, Pi Y T, LU L M, et al. Hierarchical porous active carbon from fallen leaves by synergy of K2CO3 and their supercapacitor performance. Journal of Power Sources, 2015, 299: 519-528.
DOI URL |
| [21] |
MACHAKA R, ERASMUS R M, DERRY T E. Formation of c-BN nanocrystals by He+ implantation into hBN. Diamond and Related Materials, 2010, 19(10): 1131-1134.
DOI URL |
| [22] |
CHEN D, HOLMEN A, SUI Z, et al. Carbon mediated catalysis: a review on oxidative dehydrogenation. Chinese Journal of Catalysis, 2014, 35(6): 824-841.
DOI URL |
| [23] |
ZHAO Y, YANG L, CHEN S, et al. Can boron and nitrogen co-doping improve oxygen reduction reaction activity of carbon nanotubes? Journal of the American Chemical Society, 2013, 135(4): 1201-1204.
DOI PMID |
| [24] |
SANTHOSH KUMAR M, HAMMER N, RØNNING M, et al. The nature of active chromium species in Cr-catalysts for dehydrogenation of propane: New insights by a comprehensive spectroscopic study. Journal of Catalysis, 2009, 261(1): 116-128.
DOI URL |
| [25] | LI H, ZHOU S, ZHOU Y, et al. Effect of strontium addition to Plat inum catalyst for propane dehydrogenation. China Petroleum Processing & Petrochemical Technology, 2012, 14(3): 75-82. |
| [1] | YU Yefan, XU Ling, NI Zhongbing, SHI Dongjian, CHEN Mingqing. Prussian Blue Modified Biochar: Preparation and Adsorption of Ammonia Nitrogen from Sewage [J]. Journal of Inorganic Materials, 2023, 38(2): 205-212. |
| [2] | XIAO Yao, WU Zhongjie, CUI Mei, SU Rongxin, XIE Lianke, HUANG Renliang. Co-modification of Biochar and Bentonite for Adsorption and Stabilization of Pb2+ ions [J]. Journal of Inorganic Materials, 2021, 36(10): 1083-1090. |
| [3] | ZHANG Dongshuo,CAI Hao,GAO Kaiyin,MA Zichuan. Preparation and Visible-light Photocatalytic Degradation on Metronidazole of Zn2SiO4-ZnO-biochar Composites [J]. Journal of Inorganic Materials, 2020, 35(8): 923-930. |
| [4] | DONG Lijia, WU Siying, LI Shengbo, WEI Zuofu, YANG Guo, HU Baowei. Sorption Behaviors and Mechanisms of Eu(III) on Rice Straw-derived Biochar [J]. Journal of Inorganic Materials, 2020, 35(3): 390-398. |
| [5] | WANG Hai, YANG Ningcan, QIU Muqing. Adsorption of Cr(VI) from Aqueous Solution by Biochar-clay Derived from Clay and Peanut Shell [J]. Journal of Inorganic Materials, 2020, 35(3): 301-308. |
| [6] | Guo-Hao XU, Jin-Peng YU, Hua-Sheng XU, Chun-Cheng LI, Jin-Hua HUANG, Peng-Fei WANG. Catalystic Performance of HZSM-5 Zeolite Treated by CH3COONa [J]. Journal of Inorganic Materials, 2019, 34(5): 546-552. |
| [7] | CAO Lei, DAI Peng-Cheng, LIU Dan-Dan, GU Xin, LI Liang-Jun, ZHAO Xue-Bo. Carbonyl Groups Modified Graphite Sheets Catalyze Oxidative Dehydrogenation of Propane to Propene [J]. Journal of Inorganic Materials, 2019, 34(11): 1187-1192. |
| [8] | GUO Yu, LI Dong-Xin, WU Hong-Mei, JIN Yu-Jia, ZHOU Li-Dai, CHEN Qiang-Qiang. Preparation, Characterization and Catalytic Performance of Supported Titanium Silicalite-1 Zeolite Membrane Catalyst [J]. Journal of Inorganic Materials, 2017, 32(6): 631-636. |
| [9] | QIN Zhao-Lu, LI Ding-Hua, YANG Rong-Jie. Preparation of Ammonium Polyphosphate Coated with Aluminium Hydroxide and Its Application in Polypropylene as Flame Retardant [J]. Journal of Inorganic Materials, 2015, 30(12): 1267-1272. |
| [10] | LI Hai-Yan, TAN Ye-Qiang, ZHANG Lu, CHEN Tao, SONG Yi-Hu, YE Ying, XIA Mei-Sheng. Bio-filler from Mussel Shell: Preparation and Its Effects on Polypropylene Composites Properties [J]. Journal of Inorganic Materials, 2012, 27(9): 977-983. |
| [11] | ZHAO Chun-Nian,CHENG Lai-Fei,ZHANG Li-Tong,XU Yong-Dong,LU Cui-Ying,YE Fang. In situ Kinetics Study in Chemical Vapor Deposition of Pyrocarbon from Propylene [J]. Journal of Inorganic Materials, 2008, 23(6): 1165-1170. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||