
Journal of Inorganic Materials ›› 2022, Vol. 37 ›› Issue (1): 29-37.DOI: 10.15541/jim20210547
Special Issue: 【能源环境】CO2绿色转换; 2022年度中国知网高下载论文
• TOPICAL SECTION: Green Conversion of CO2 (Contributing Editor: OUYANG Shuxin, WANG Wenzhong) • Previous Articles Next Articles
GUO Lina( ), HE Xuebing, LYU Lin, WU Dan, YUAN Hong(
), HE Xuebing, LYU Lin, WU Dan, YUAN Hong( )
)
Received:2021-09-04
Revised:2021-10-22
Published:2022-01-20
Online:2021-11-01
Contact:
YUAN Hong, professor. E-mail: yuanhong@mail.ccnu.edu.cn
About author:GUO Lina (1998-), female, Master candidate. E-mail: gln@mails.ccnu.edu.cn
Supported by:CLC Number:
GUO Lina, HE Xuebing, LYU Lin, WU Dan, YUAN Hong. Modulation of CuO Surface Properties for Selective Electrocatalytic Reduction of CO2 to HCOOH[J]. Journal of Inorganic Materials, 2022, 37(1): 29-37.
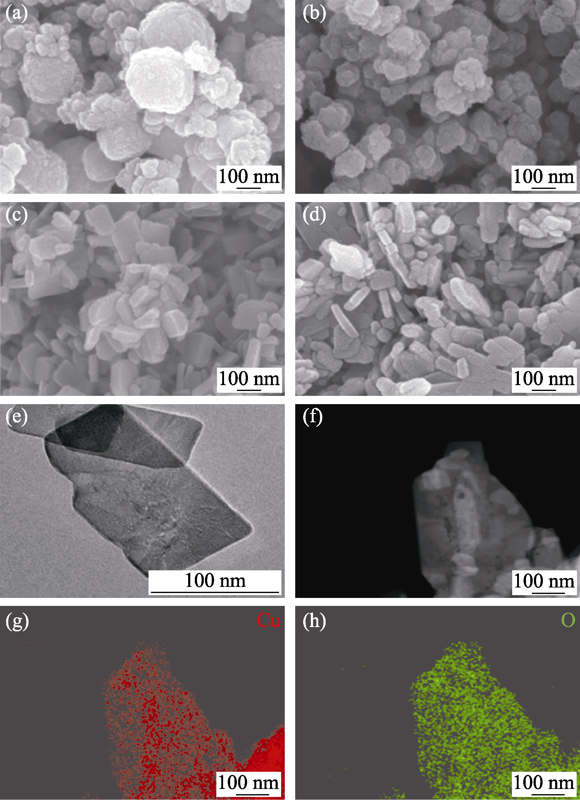
Fig. 1 Morphologies and element composition of samples FESEM images of (a) Cu2O, (b) Cu2S, (c) CuO-FO and (d) CuO-FS; (e) TEM image of CuO-FS; (f) HAADF image of the CuO-FS; (g, h) Corresponding EDS elemental mapping
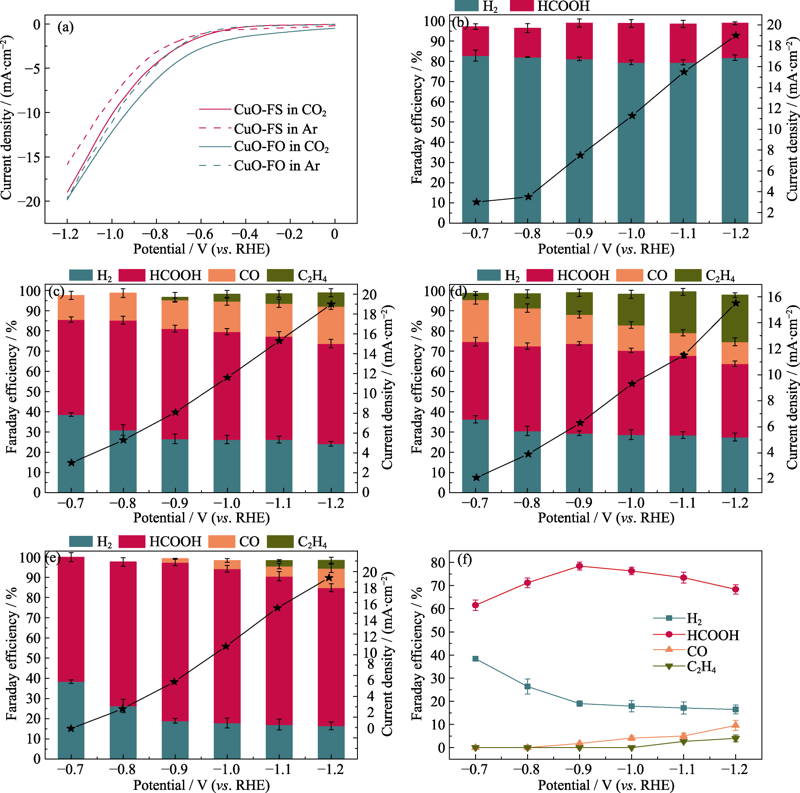
Fig. 4 (a) Cathodic polarization curves of CuO-FO and CuO-FS in 0.1 mol/L KHCO3 electrolyte saturated with CO2/Ar, FE of all products and current density over (b) Cu2S, (c) Cu2O, (d) CuO-FO, and (e) CuO-FS, and (f) FE of all products of CuO-FS tested at different voltages Colorful figures are available on website
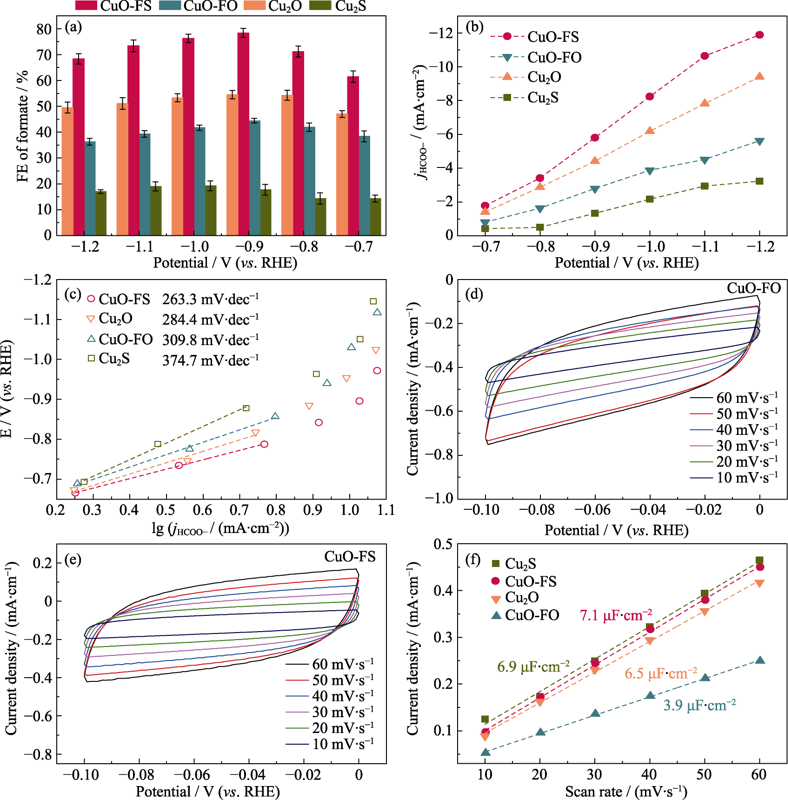
Fig. 5 (a) FE, (b) partial current densities, (c) Tafel plots of HCOOH over Cu2S, Cu2O, CuO-FO and CuO-FS; (d,e) CV curves of (d) CuO-FO and (e) CuO-FS at various test voltage scan rates, and (f) current density of four samples at various test voltage scan rates Colorful figures are available on website
| [1] | VASILEFF A, YAO Z, SHI Z Q. Carbon solving carbon's problems: recent progress of nanostructured carbon-based catalysts for the electrochemical reduction of CO2. Advanced Energy Materials, 2017, 7(21):724-761. |
| [2] |
PETERS, GLEN, ANDERSON. et al. The trouble with negative emissions. Science, 2016, 354(6309):182-183.
DOI URL |
| [3] | ZHANG S, ZHAO S, QU D, et al. Electrochemical reduction of CO2 toward C2 valuables on Cu@Ag core-shell tandem catalyst with tunable shell thickness. Small, 2021, 2102293. |
| [4] |
XU K, NING S, CHEN H, et al. Plum pudding-like electrocatalyst of N-doped SnOx@Sn loaded on carbon matrix to construct photovoltaic CO2 reduction system with solar-to-fuel efficiency of 11.3%. Solar RRL, 2020, 4(7):2000116.
DOI URL |
| [5] |
QI Y, SONG L, OUYANG S, et al. Photoinduced defect engineering: enhanced photothermal catalytic performance of 2D black In2O(3-x) nanosheets with bifunctional oxygen vacancies. Advanced Materials, 2020, 32(6):1903915.
DOI URL |
| [6] |
LI R, LI Y, LI Z, et al. A metal-segregation approach to generate CoMn alloy for enhanced photothermal conversion of syngas to light olefins. Solar RRL, 2020, 5(2):2000488.
DOI URL |
| [7] |
ZHANG C, CAO C, ZHANG Y, et al. Unraveling the role of zinc on bimetallic Fe5C2-ZnO catalysts for highly selective carbon dioxide hydrogenation to high carbon α-olefins. ACS Catalysis, 2021, 11(4):2121-2133.
DOI URL |
| [8] |
PADILLA M A, LU Q, BATURINA O A. CO2 electroreduction to hydrocarbons on carbon-supported Cu nanoparticles. ACS catalysis, 2014, 4(10):3682-3695.
DOI URL |
| [9] |
DUAN X, XU J, WEI Z, et al. Metal-free carbon materials for CO2 electrochemical reduction. Advanced Materials, 2017, 29:1701784.
DOI URL |
| [10] |
JIN S, HAO Z, ZHANG K, et al. Advances and challenges for electrochemical reduction of CO2 to CO: from fundamental to industrialization. Angewandte Chemie International Edition, 2021, 60:2-24.
DOI URL |
| [11] |
GU J, HSU C S, BAI L, et al. Atomically dispersed Fe3+ sites catalyze efficient CO2 electroreduction to CO. Science, 2019, 364(6445):1091-1094.
DOI URL |
| [12] | LIU G, LI Z, SHI J, et al. Black reduced porous SnO2 nanosheets for CO2 electroreduction with high formate selectivity and low overpotential. Applied Catalysis B: Environmental, 2019, 260:118-134. |
| [13] | LIN L, LIU T, XIAO J, et al. Enhancing CO2 electroreduction to methane with cobalt phthalocyanine and zinc-nitrogen-carbon tandem catalyst. Angewandte Chemie, 2020, 59(50):22408-22413. |
| [14] |
YANG D, ZHU Q, CHEN C, et al. Selective electroreduction of carbon dioxide to methanol on copper selenide nanocatalysts. Nature Communications, 2019, 10(1):1-9.
DOI URL |
| [15] |
DINH C T, BURDYNY T, KIBRIA M, et al. CO2 electroreduction to ethylene via hydroxide-mediated copper catalysis at an abrupt interface. Science, 2018, 360(6390):783-787.
DOI URL |
| [16] |
ZANG D, LI Q, DAI G, et al. Interface engineering of Mo8/Cu heterostructures toward highly selective electrochemical reduction of carbon dioxide into acetate. Applied Catalysis B: Environmental, 2020, 281:119426.
DOI URL |
| [17] |
LV X, SHANG L, ZHOU S, et al. Electron-deficient Cu sites on Cu3Ag1 catalyst promoting CO2 electroreduction to alcohols. Advanced Energy Materials, 2020, 10(37):2001987.
DOI URL |
| [18] |
ZU X, LI X, WEI L, et al. Efficient and robust carbon dioxide electroreduction enabled by atomically dispersed Snδ+ sites. Advanced Materials, 2019, 31(15):1808135.
DOI URL |
| [19] |
SHI Y, JI Y, LONG J, et al. Unveiling hydrocerussite as an electrochemically stable active phase for efficient carbon dioxide electroreduction to formate. Nature Communications, 2020, 11(1):3415.
DOI URL |
| [20] |
ZHANG A, LIANG Y, LI H, et al. In-situ surface reconstruction of InN nanosheets for efficient CO2 electroreduction into formate. Nano Letters, 2020, 20(11):8229-8235.
DOI URL |
| [21] | SUN J, ZHENG W, LYU S, et al. Bi/Bi2O3 nanoparticles supported on N-doped reduced graphene oxide for highly efficient CO2 electroreduction to formate. Chinese Chemical Letters, 31(6):8229-8235. |
| [22] |
NITOPI S, BERTHEUSSEN E, SCOTT S B, et al. Progress and perspectives of electrochemical CO2 reduction on copper in aqueous electrolyte. Chemical Reviews, 2019, 119(12):7610-7672.
DOI URL |
| [23] |
LV L, HE X, WANG J, et al. Charge localization to optimize reactant adsorption on KCu7S4/CuO interfacial structure toward selective CO2 electroreduction. Applied Catalysis B: Environmental, 2021, 298:120531.
DOI URL |
| [24] |
XIE H, WANG T, LIANG J, et al. Cu-based nanocatalysts for electrochemical reduction of CO2. Nano Today, 2018, 21:41-54.
DOI URL |
| [25] |
WANG X, WANG Z, ZHUANG T T, et al. Efficient upgrading of CO to C3 fuel using asymmetric C-C coupling active sites. Nature Communications, 2019, 10(1):5186.
DOI URL |
| [26] |
MA M, DJANASHVILI K, SMITH W A. Selective electrochemical reduction of CO2 to CO on CuO-derived Cu nanowires. Physical Chemistry Chemical Physics, 2015, 17(32):20861-20867.
DOI URL |
| [27] |
LIU G, LI Z, SHI J, et al. Black reduced porous SnO2 nanosheets for CO2 electroreduction with high formate selectivity and low overpotential. Applied Catalysis B: Environmental, 2019, 260:118134.
DOI URL |
| [28] |
CHOU T C, CHANG C C, YU H L, et al. Controlling the oxidation state of Cu electrode and reaction intermediates for electrochemical CO2 reduction to ethylene. Journal of the American Chemical Society, 2020, 142:2857-2867.
DOI URL |
| [29] |
DAIYAN R, SAPUTERA W H, ZHANG Q, et al. 3D heterostructured copper electrode for conversion of carbon dioxide to alcohols at low overpotentials. Advanced Sustainable Systems, 2019, 3(1):1800064.
DOI URL |
| [1] | WANG Hongli, WANG Nan, WANG Liying, SONG Erhong, ZHAO Zhankui. Hydrogen Generation from Formic Acid Boosted by Functionalized Graphene Supported AuPd Nanocatalysts [J]. Journal of Inorganic Materials, 2022, 37(5): 547-553. |
| [2] | SHI Zhang-Yu, LI Quan, TANG Song-Chao, QIAN Jun, PAN Yong-Kang, WEI Jie. Surface Modification on Property of Mesoporous Calcium Magnesium Silicate/Polyetheretherketone Composites [J]. Journal of Inorganic Materials, 2018, 33(1): 67-74. |
| [3] | HUANG Xiao-Ling, LIN Zhou, LIAN Xing-Yi, ZHANG Xiao-Feng, LIN Shen. Preparation and Electrocatalytic Properties of Pd/PMo12-GN Composite towards Formic Acid Oxidation [J]. Journal of Inorganic Materials, 2014, 29(7): 722-728. |
| [4] | JIANG Hong,FENG Lan-Ying,ZHU Hong,GUO Zhi-Jun,ZHANG Xin-Wei. Effect of Adding Fe on the Performances of Pd/C Catalyst [J]. Journal of Inorganic Materials, 2008, 23(4): 847-850. |
| [5] | ZHANG Yao-Jun,ZHANG,Li. Synthesis of Composite Material CdS/Al-HMS and Hydrogen Production by Photocatalytic Pollutant Degradation under Visible Light Irradiation [J]. Journal of Inorganic Materials, 2008, 23(1): 66-70. |
| [6] | SHANG Jing,XU Zi-Li,DU Yao-Guo,LI Jing-Min. Structure, Surface Properties and Photocatalytic Activity of TiO2 Nanoparticles [J]. Journal of Inorganic Materials, 2001, 16(6): 1211-1216. |
| [7] | SUN Jing, GAO Lian, GUO Jing-Kun. Surface Properties of Silicon Carbide Powders and Rheological Properties of Their Slurries [J]. Journal of Inorganic Materials, 2000, 15(3): 426-430. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||