
Journal of Inorganic Materials ›› 2021, Vol. 36 ›› Issue (10): 1053-1058.DOI: 10.15541/jim20210044
Special Issue: 【虚拟专辑】碳中和(2020~2021); 【能源环境】CO2绿色转换
• RESEARCH ARTICLE • Previous Articles Next Articles
LIU Qiang( ), DING Jie(
), DING Jie( ), JI Guojing, HU Juanmin, GU Hao, ZHONG Qin(
), JI Guojing, HU Juanmin, GU Hao, ZHONG Qin( )
)
Received:2021-01-25
Revised:2021-03-30
Published:2021-10-20
Online:2021-04-25
Contact:
DING Jie, lecturer. E-mail: tonlyjding@njust.edu.cn; ZHONG Qin, professor. E-mail: zq304@njust.edu.cn
About author:LIU Qiang(1996-), male, Master candidate. E-mail: qiaangliu@163.com
Supported by:CLC Number:
LIU Qiang, DING Jie, JI Guojing, HU Juanmin, GU Hao, ZHONG Qin. Fe-Co-K/ZrO2 Catalytic Performance of CO2 Hydrogenation to Light Olefins[J]. Journal of Inorganic Materials, 2021, 36(10): 1053-1058.
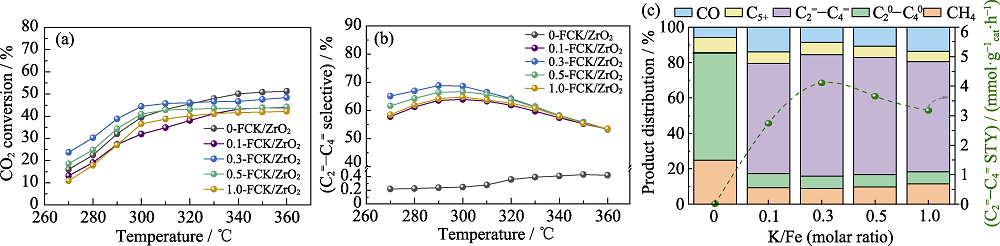
Fig. 1 CO2 conversion (a) and light olefins selectivity (b) of CO2 hydrogenation by x-FCK/ZrO2 at different temperatures and catalytic production distribution of x-FCK/ZrO2 at 300 ℃(c) Colorful images are available on website
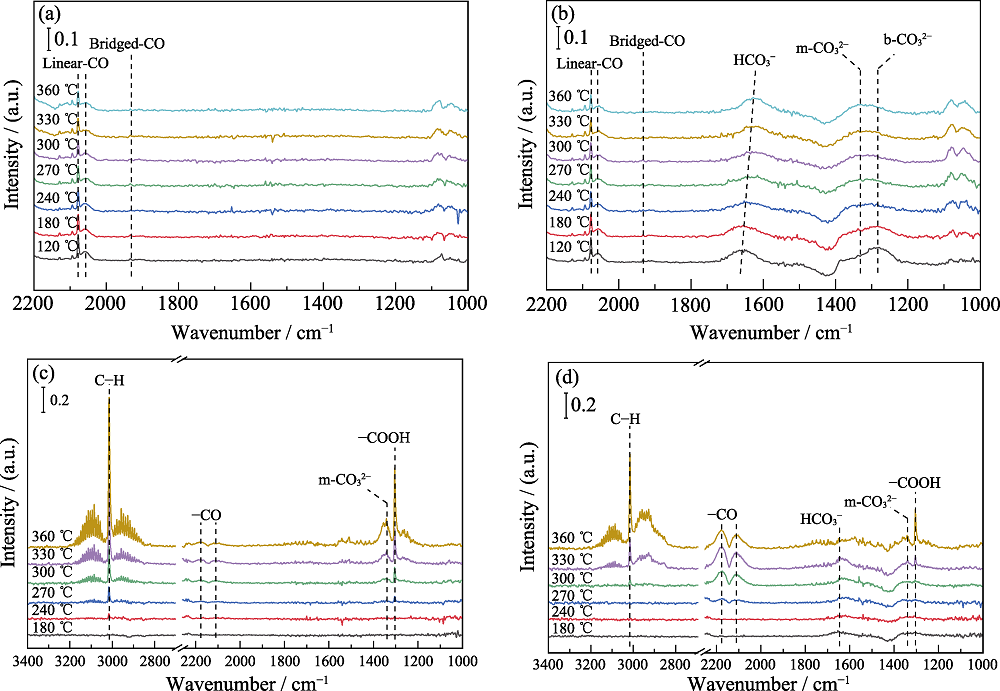
Fig. 8 In-situ DRIFTS of 0-FCK/ZrO2 (a) and 0.3-FCK/ZrO2 (b) with pure CO2 introduced, and in-situ DRIFTS of 0-FCK/ZrO2 (c) and 0.3-FCK/ZrO2 (d) with CO2 and H2 introduced
| [1] |
CHEN S Q, LÜ G X. CO2 methanation over Ru/TiO2 catalysts under UV irradiation and heating. Journal of Inorganic Materials, 2014, 29(12):1287-1293.
DOI URL |
| [2] |
JIAO F, LI J J, PAN X L, et al. Selective conversion of syngas to light olefins. Science, 2016, 351(6277):1065-1068.
DOI URL |
| [3] |
TORRES G H, BITTER J H, KHARE C B, et al. Supported iron nanoparticles as catalysts for sustainable production of lower olefins. Science, 2012, 335(6070):835-838.
DOI URL |
| [4] |
AMOYAL M, VIDRUK N R, LANDAU M V, et al. Effect of potassium on the active phases of Fe catalysts for carbon dioxide conversion to liquid fuels through hydrogenation. Journal of Catalysis, 2017, 348:29-39.
DOI URL |
| [5] | LI W H, ZHANG A F, JIANG X, et al. The anti-sintering catalysts: Fe-Co-Zr polymetallic fibers for CO2 hydrogenation to C2=-C4=- rich hydrocarbons. Journal of CO2 Utilization, 2018, 23:219-225. |
| [6] | SATTHAWONG R, KOIZUMI N, SONG C S, et al. Bimetallic Fe-Co catalysts for CO2 hydrogenation to higher hydrocarbons. Journal of CO2 Utilization, 2013, 34:102-106. |
| [7] |
NUMPILAI T, CHANLEK N, POO A Y, et al. Tuning interactions of surface-adsorbed species over Fe-Co/K-Al2O3 catalyst by different K contents: selective CO2 hydrogenation to light olefins. ChemCatChem, 2020, 12(12):3306-3320.
DOI URL |
| [8] |
DING J, HUANG L, GONG W B, et al. CO2 hydrogenation to light olefins with high-performance Fe0.30Co0.15Zr0.45K0.10O1.63. Journal of Catalysis, 2019, 377:224-232.
DOI URL |
| [9] |
SHAFER W D, JACOBS G, GRAHAM U M, et al. Increased CO2 hydrogenation to liquid products using promoted iron catalysts. Journal of Catalysis, 2019, 369:239-248.
DOI URL |
| [10] |
NUMPILAI T, WITOON T, CHANLEK N, et al. Structure-activity relationships of Fe-Co/K-Al2O3 catalysts calcined at different temperatures for CO2 hydrogenation to light olefins. Applied Catalysis A: General, 2017, 547:219-229.
DOI URL |
| [11] |
LI X, LIN J, LI L, et al. Controlling CO2 hydrogenation selectivity by metal-supported electron transfer. Angew. Chem. Int. Ed., 2020, 59(45):19983-19989.
DOI URL |
| [12] |
BRODEN G, BONZEL H P. Potassium adsorption on Fe(110). Surface Science, 1979, 84(1):106-120.
DOI URL |
| [13] |
YAN B, WU Q Y, CEN J J, et al. Highly active subnanometer Rh clusters derived from Rh-doped SrTiO3 for CO2 reduction. Applied Catalysis B: Environmental, 2018, 237:1003-1011.
DOI URL |
| [14] |
SHAO C P, CHEN M S. In situ. FT-IR study on CO2 hydrogenation over SiO2-supported PtM (M=Cr, Mo, W) complex catalysts. Journal of Molecular Catalysis A: Chemical, 2001, 170(1):245-249.
DOI URL |
| [15] |
WU H C, CHEN T C, WU J H, et al. Influence of sodium- modified Ni/SiO2 catalysts on the tunable selectivity of CO2 hydrogenation: effect of the CH4 selectivity, reaction pathway and mechanism on the catalytic reaction. J. Colloid Interface Sci., 2021, 586:514-527.
DOI URL |
| [16] | XU S S, CHANSAI S, XU S J, et al. CO poisoning of Ru catalysts in CO2 hydrogenation under thermal and plasma conditions: a combined kinetic and diffuse reflectance infrared fourier transform spectroscopy-mass spectrometry study. ACS Catalysis, 2020, 10(21):12828-12840. |
| [17] | ZHAO F G, FAN L L, XU K J, et al. Hierarchical sheet-like Cu/Zn/Al nanocatalysts derived from LDH/MOF composites for CO2 hydrogenation to methanol. Journal of CO2Utilization, 2019, 33:222-232. |
| [18] |
NOIROJ K, INTARAPONG P, LUENGNARUEMITCHAI A, et al. A comparative study of KOH/Al2O3 and KOH/NaY catalysts for biodiesel production via transesterification from palm oil. Renewable Energy, 2009, 34(4):1145-1150.
DOI URL |
| [19] |
HUYNH H L, ZHU J, ZHANG G H, et al. Promoting effect of Fe on supported Ni catalysts in CO2 methanation by in situ DRIFTS and DFT study. Journal of Catalysis, 2020, 392:266-277.
DOI URL |
| [20] |
WANG F, HE S, CHEN H, et al. Active site dependent reaction mechanism over Ru/CeO2 catalyst toward CO2 methanation. J. Am. Chem. Soc., 2016, 138(19):6298-6305.
DOI URL |
| [21] | HAN S J, HWANG S M, PARK H G, et al. Identification of active sites for CO2 hydrogenation in Fe catalysts by first-principles microkinetic modelling. Journal of Materials Chemistry A, 2020, 8(26):13014-13023. |
| [22] | LIU X L, CAO C X, TIAN P F, et al. Resolving CO2 activation and hydrogenation pathways over iron carbides from DFT investigation. Journal of CO2 Utilization, 2020, 38:10-15. |
| [23] |
GU H, DING J, ZHONG Q, et al. Promotion of surface oxygen vacancies on the light olefins synthesis from catalytic CO2 hydrogenation over Fe-K/ZrO2 catalysts. International Journal of Hydrogen Energy, 2019, 44(23):11808-11816.
DOI URL |
| [24] |
YANG X M, WEI Y, SU Y L, et al. Characterization of fused Fe-Cu based catalyst for higher alcohols synthesis and DRIFTS investigation of TPSR. Fuel Processing Technology, 2010, 91(9):1168-1173.
DOI URL |
| [25] | YANG C, ZHAO H, HOU Y, et al. Fe5C2 nanoparticles: a facile bromide-induced synthesis and as an active phase for Fischer- Tropsch synthesis. J. Am. Chem. Soc., 2012, 134(38):15814-15821. |
| [26] | WANG H Z, NIE X W, CHEN Y G, et al. Facet effect on CO2 adsorption, dissociation and hydrogenation over Fe catalysts: insight from DFT. Journal of CO2 Utilization, 2018, 26:160-170. |
| [1] | TANG Xiao-Hua, LI Hui, YANG Ai-Mei, ZHA Fei, CHANG Yue. Imidazole and Nickel (II) Modified SAPO-34 and Its Catalytic Activity in CO2 Hydrogenation to Ethylene [J]. Journal of Inorganic Materials, 2017, 32(11): 1209-1214. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||