
Journal of Inorganic Materials ›› 2021, Vol. 36 ›› Issue (4): 372-378.DOI: 10.15541/jim20200374
Special Issue: 【结构材料】高熵陶瓷
• RESEARCH PAPER • Previous Articles Next Articles
ZHANG Fengnian( ), GUO Meng, MIAO Yang(
), GUO Meng, MIAO Yang( ), GAO Feng, CHENG Chufei, CHENG Fuhao, LIU Yufeng
), GAO Feng, CHENG Chufei, CHENG Fuhao, LIU Yufeng
Received:2020-07-06
Revised:2020-08-28
Published:2021-04-20
Online:2021-04-19
Contact:
MIAO Yang, associate professor. E-mail: miaoyang@tyut.edu.cn
About author:ZHANG Fengnian(1998-), male, Master candidate. E-mail: zhangfn1998@163.com
Supported by:CLC Number:
ZHANG Fengnian, GUO Meng, MIAO Yang, GAO Feng, CHENG Chufei, CHENG Fuhao, LIU Yufeng. Preparation and Sintering Behavior of High Entropy Ceramic (Zr1/7Hf1/7Ce1/7Y2/7La2/7)O2-δ[J]. Journal of Inorganic Materials, 2021, 36(4): 372-378.
| Oxides | Crystal structure | Space group (number) | CN | rc /nm |
|---|---|---|---|---|
| ZrO2 | Cubic | Fm-3m (225) | 8 | 0.084 |
| HfO2 | Monoclinic | P21/c (14) | 8 | 0.083 |
| CeO2 | Fluorite | Fm-3m (225) | 8 | 0.097 |
| Y2O3 | Bixbyite | Ia-3 (206) | 6 | 0.090 |
| La2O3 | Trigonal | P-3m (164) | 6 | 0.1032 |
Table 1 Crystal structures, space groups (number), cation coordination numbers (CN) and corresponding cationic radii (rc) of selected oxides[42]
| Oxides | Crystal structure | Space group (number) | CN | rc /nm |
|---|---|---|---|---|
| ZrO2 | Cubic | Fm-3m (225) | 8 | 0.084 |
| HfO2 | Monoclinic | P21/c (14) | 8 | 0.083 |
| CeO2 | Fluorite | Fm-3m (225) | 8 | 0.097 |
| Y2O3 | Bixbyite | Ia-3 (206) | 6 | 0.090 |
| La2O3 | Trigonal | P-3m (164) | 6 | 0.1032 |
| Sample | Process | BPR | Speed /(r·min-1) | Time /h | Disperser | Desiccation | Energy |
|---|---|---|---|---|---|---|---|
| A | Wet milling | 6 : 1 | 250 | 6 | Ethanol | 60 ℃ /24 h | Low |
| B | Dry milling | 10 : 1 | 400 | 40 | - | - | High |
Table 2 Process, ball-to-powder-ratio (BPR), speed, time and others for raw material preparation
| Sample | Process | BPR | Speed /(r·min-1) | Time /h | Disperser | Desiccation | Energy |
|---|---|---|---|---|---|---|---|
| A | Wet milling | 6 : 1 | 250 | 6 | Ethanol | 60 ℃ /24 h | Low |
| B | Dry milling | 10 : 1 | 400 | 40 | - | - | High |
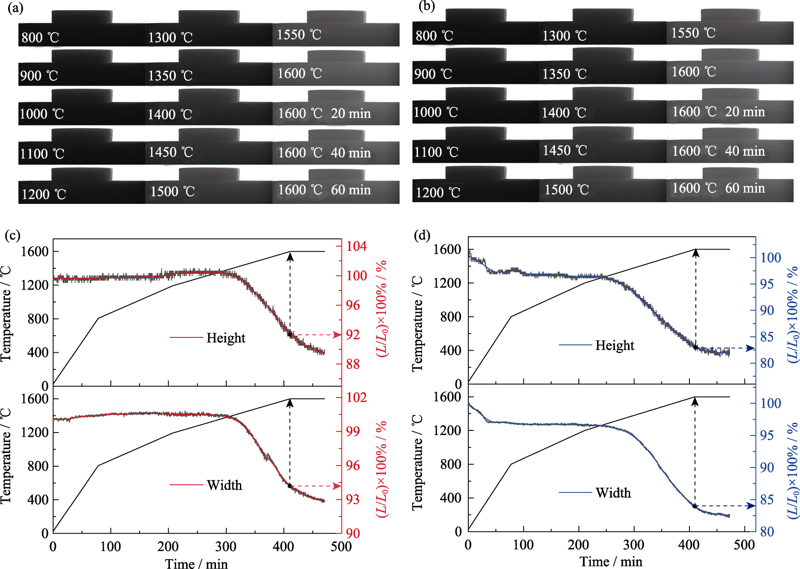
Fig. 8 Digital camera photographs of different pellets sintered at different temperatures and temper ature/linear shrinkage of different pellets as a function of time (a,c) Pellet A; (b,d) Pellet B
| [1] | MIRACLE D B, SENKOV O N. A critical review of high entropy alloys and related concepts. Acta Materialia, 2017,122:448-511. |
| [2] | ZHU J M, FU H M, ZHANG F, et al. Synthesis and properties of multiprincipal component AlCoCrFeNiSix alloys. Materials Science and Engineering: A, 2010,527(27):7210-7214. |
| [3] | SZKLARZ Z, LEKKI J, BOBROWSKI P, et al. The effect of SiC nanoparticles addition on the electrochemical response of mechanically alloyed CoCrFeMnNi high entropy alloy. Materials Chemistry and Physics, 2018,215:385-392. |
| [4] | TSAI M, WANG C W, TSAI C W, et al. Thermal stability and performance of NbSiTaTiZr high-entropy alloy barrier for copper metallization. Journal of The Electrochemical Society, 2011,158(11):H1161-H1165. |
| [5] | ROST C M, SACHET E, BORMAN T, et al. Entropy-stabilized oxides. Nature Communications, 2015,6(1):8485. |
| [6] | WEI X F, LIU J X, LI F, et al. High entropy carbide ceramics from different starting materials. Journal of the European Ceramic Society, 2019,39(10):2989-2994. |
| [7] | JIN T, SANG X H, UNOCIC R R, et al. Mechanochemical-assisted synthesis of high-entropy metal nitride via a soft urea strategy. Advanced Materials, 2018,30(23):1707512. |
| [8] | LIU J X, SHEN X Q, WU Y, et al. Mechanical properties of hot-pressed high-entropy diboride-based ceramics. Journal of Advanced Ceramics, 2020,9(4):503-510. |
| [9] | QIN Y, LIU J X, LI F, et al. A high entropy silicide by reactive spark plasma sintering. Journal of Advanced Ceramics, 2019,8(1):148-152. |
| [10] |
CHEN X Q, WU Y Q. High-entropy transparent fluoride laser ceramics. Journal of the American Ceramic Society, 2020,103(2):750-756.
DOI URL |
| [11] |
ZHANG R Z, GUCCI F, ZHU H Y, et al. Data-driven design of ecofriendly thermoelectric high-entropy sulfides. Inorganic Chemistry, 2018,57(20):13027-13033.
DOI URL PMID |
| [12] | DJENADIC R, SARKAR A, CLEMENS O, et al. Multicomponent equiatomic rare earth oxides. Materials Research Letters, 2017,5(2):102-109. |
| [13] | MAO A Q, XIANG H Z, ZHANG Z G, et al. Solution combustion synthesis and magnetic property of rock-salt (Co0.2Cu0.2Mg0.2Ni0.2Zn0.2)O high-entropy oxide nanocrystalline powder. Journal of Magnetism and Magnetic Materials, 2019,484:245-252. |
| [14] | XING Q W, XIA S Q, YAN X H, et al. Mechanical properties and thermal stability of (NbTiAlSiZr)Nx high-entropy ceramic films at high temperatures. Journal of Materials Research, 2018,33(19):3347-3354. |
| [15] | CHEN L, WANG K, SU W T, et al. Research progress of transition metal non-oxide high-entropy ceramics. Journal of Inorganic Materials, 2020,35(7):748-758. |
| [16] | CHEN H, QIU N, WU B Z, et al. Tunable pseudocapacitive contribution by dimension control in nanocrystalline-constructed (Mg0.2Co0.2Ni0.2Cu0.2Zn0.2)O solid solutions to achieve superior lithium-storage properties. RSC Advances, 2019,9(50):28908-28915. |
| [17] | BÉRARDAN D, FRANGER S, DRAGOE D, et al. Colossal dielectric constant in high entropy oxides. Physica Status Solidi, 2016,10(4):328-333. |
| [18] | ZHANG J J, YAN J Q, CALDER S, et al. Long-range antiferromagnetic order in a rocksalt high entropy oxide. Chemistry of Materials, 2019,31(10):3705-3711. |
| [19] | BéRARDAN D, FRANGER S, MEENA A K, et al. Room temperature lithium superionic conductivity in high entropy oxides. Journal of Materials Chemistry A, 2016,4(24):9536-9541. |
| [20] | CHEN H, FU J, ZHANG P F, et al. Entropy-stabilized metal oxide solid solutions as CO oxidation catalysts with high-temperature stability. Journal of Materials Chemistry A, 2018,6(24):11129-11133. |
| [21] | CHEN H, LIN W W, ZHANG Z H, et al. Mechanochemical synthesis of high entropy oxide materials under ambient conditions: dispersion of catalysts via entropy maximization. ACS Materials Letters, 2019,1(1):83-88. |
| [22] | SARKAR A, DJENADIC R, WANG D, et al. Rare earth and transition metal based entropy stabilised perovskite type oxides. Journal of the European Ceramic Society, 2018,38(5):2318-2327. |
| [23] | PU Y P, ZHANG Q W, LI R, et al. Dielectric properties and electrocaloric effect of high-entropy (Na0.2Bi0.2Ba0.2Sr0.2Ca0.2)TiO3 ceramic. Applied Physics Letters, 2019,115(22):223901. |
| [24] | LIU J, REN K, MA C Y, et al. Dielectric and energy storage properties of flash-sintered high-entropy (Bi0.2Na0.2K0.2Ba0.2Ca0.2)TiO3 ceramic. Ceramics International, 2020,46(12):20576-20581. |
| [25] | EDALATI P, WANG Q, RAZAVI-KHOSROSHAHI H, et al. Photocatalytic hydrogen evolution on a high-entropy oxide. Journal of Materials Chemistry A, 2020,8(7):3814-3821. |
| [26] |
WANG T, CHEN H, YANG Z, et al. High-entropy perovskite fluorides: a new platform for oxygen evolution catalysis. Journal of the American Chemical Society, 2020,142(10):4550-4554.
URL PMID |
| [27] | FRACCHIA M, MANZOLI M, ANSELMI-TAMBURINI U, et al. A new eight-cation inverse high entropy spinel with large configurational entropy in both tetrahedral and octahedral sites: Synthesis and cation distribution by X-ray absorption spectroscopy. Scripta Materialia, 2020,188:26-31. |
| [28] | WANG D, JIANG S D, DUAN C Q, et al. Spinel-structured high entropy oxide (FeCoNiCrMn)3O4 as anode towards superior lithium storage performance. Journal of Alloys and Compounds, 2020,844:156158. |
| [29] | MAO A Q, QUAN F, XIANG H Z, et al. Facile synthesis and ferrimagnetic property of spinel (CoCrFeMnNi)3O4 high-entropy oxide nanocrystalline powder. Journal of Molecular Structure, 2019,1194:11-18. |
| [30] | WANG J B, STENZEL D, AZMI R, et al. Spinel to rock-salt transformation in high entropy oxides with Li incorporation. Electrochem, 2020,1(1):60-74. |
| [31] | LI F, ZHOU L, LIU J X, et al. High-entropy pyrochlores with low thermal conductivity for thermal barrier coating materials. Journal of Advanced Ceramics, 2019,8(4):576-582. |
| [32] | CHEN H, ZHAO Z F, XIANG H M, et al. High entropy (Y0.2Yb0.2Lu0.2Eu0.2Er0.2)3Al5O12: a novel high temperature stable thermal barrier material. Journal of Materials Science & Technology, 2020,48:57-62. |
| [33] | ZHAO Z F, CHEN H, XIANG H M, et al. (La0.2Ce0.2Nd0.2Sm0.2Eu0.2)PO4: a high-entropy rare-earth phosphate monazite ceramic with low thermal conductivity and good compatibility with Al2O3. Journal of Materials Science & Technology, 2019,35(12):2892-2896. |
| [34] | CHEN H, XIANG H M, DAI F Z, et al. High entropy (Yb0.25Y0.25Lu0.25Er0.25)2SiO5 with strong anisotropy in thermal expansion. Journal of Materials Science & Technology, 2020,36:134-139. |
| [35] | VINNIK D A, TROFIMOV E A, ZHIVULIN V E, et al. The new extremely substituted high entropy (Ba,Sr,Ca,La)Fe6-x (Al,Ti,Cr,Ga,In,Cu,W)xO19 microcrystals with magnetoplumbite structure. Ceramics International, 2020,46(7):9656-9660. |
| [36] |
VINNIK D A, ZHIVULIN V E, TROFIMOV E A, et al. Extremely polysubstituted magnetic material based on magnetoplumbite with a hexagonal structure: synthesis, structure, properties, prospects. Nanomaterials (Basel), 2019,9(4):559.
DOI URL |
| [37] | SKINNER S J, KILNER J A. Oxygen ion conductors. Materials Today, 2003,6(3):30-37. |
| [38] |
SACHKOV V I, NEFEDOV R A, AMELICHKIN I V. High entropy oxide systems based on rare earth elements. IOP Conference Series: Materials Science and Engineering, 2019,597:012005.
DOI URL |
| [39] | PIANASSOLA M, LOVEDAY M, MCMURRAY J W, et al. Solid-state synthesis of multicomponent equiatomic rare-earth oxides. Journal of the American Ceramic Society, 2020,103(4):2908-2918. |
| [40] |
SARKAR A, LOHO C, VELASCO L, et al. Multicomponent equiatomic rare earth oxides with a narrow band gap and associated praseodymium multivalency. Dalton Trans., 2017,46(36):12167-12176.
DOI URL PMID |
| [41] | GILD J, SAMIEE M, BRAUN J L, et al. High-entropy fluorite oxides. Journal of the European Ceramic Society, 2018,38(10):3578-3584. |
| [42] | SHANNON R D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallographica Section A, 1976,32(5):751-767. |
| [43] |
WRIGHT A J, WANG Q Y, HUANG C Y, et al. From high-entropy ceramics to compositionally-complex ceramics: a case study of fluorite oxides. Journal of the European Ceramic Society, 2020,40(5):2120-2129.
DOI URL |
| [44] | LIU Y C, JIA D C, ZHOU Y, et al. Zn0.1Ca0.1Sr0.4Ba0.4ZrO3: a non-equimolar multicomponent perovskite ceramic with low thermal conductivity. Journal of the European Ceramic Society, 2020,40(15):6272-6277. |
| [45] |
ARTINI C, PANI M, CARNASCIALI M M, et al. Structural features of Sm- and Gd-doped ceria studied by synchrotron X-ray diffraction and μ-Raman spectroscopy. Inorganic Chemistry, 2015,54(8):4126-4137.
DOI URL PMID |
| [46] | TOBY B H. EXPGUI, a graphical user interface for GSAS. Journal of Applied Crystallography, 2001,34(2):210-213. |
| [47] | CHEN K P, PEI X T, TANG L, et al. A five-component entropy-stabilized fluorite oxide. Journal of the European Ceramic Society, 2018,38(11):4161-4164. |
| [48] |
DRAGOE N, BéRARDAN D. Order emerging from disorder. Science, 2019,366(6465):573.
URL PMID |
| [49] | CHEN H, XIANG H M, DAI F Z, et al. High porosity and low thermal conductivity high entropy (Zr0.2Hf0.2Ti0.2Nb0.2Ta0.2)C. Journal of Materials Science & Technology, 2019,35(8):1700-1705. |
| [50] | WRIGHT A J, WANG Q Y, KO S T, et al. Size disorder as a descriptor for predicting reduced thermal conductivity in medium- and high-entropy pyrochlore oxides. Scripta Materialia, 2020,181:76-81. |
| [51] | KURODA Y, HAMANO H, MORI T, et al. Specific adsorption behavior of water on a Y2O3 surface. Langmuir, 2000,16(17):6937-6947. |
| [52] | SPIRIDIGLIOZZI L, FERONE C, CIOFFI R, et al. Entropy-stabilized oxides owning fluorite structure obtained by hydrothermal treatment. Materials, 2000,16(17):6937-6947. |
| [53] | CHEN H, ZHAO Z F, XIANG H M, et al. Effect of reaction routes on the porosity and permeability of porous high entropy (Y0.2Yb0.2Sm0.2Nd0.2Eu0.2)B6 for transpiration cooling. Journal of Materials Science & Technology, 2020,38:80-85. |
| [54] | CUI S F, YANG W S, QIAN Z N. Research thermal decomposition fo lanthanum hydroxide by thermogravimetry. Chemical Journal of Chinese University, 1987,8(3):271-272. |
| [55] | SURYANARAYANA C. Mechanical alloying and milling. Progress in Materials Science, 2001,46(1/2):1-184. |
| [56] |
HARRINGTON T J, GILD J, SARKER P, et al. Phase stability and mechanical properties of novel high entropy transition metal carbides. Acta Materialia, 2019,166:271-280.
DOI URL |
| [1] | LI Wangguo, LIU Dianguang, WANG Kewei, MA Baisheng, LIU Jinling. High Entropy Oxide Ceramics (MgCoNiCuZn)O: Flash Sintering Synthesis and Properties [J]. Journal of Inorganic Materials, 2022, 37(12): 1289-1294. |
| [2] | WANG Yiliang, AI Yunlong, YANG Shuwei, LIANG Bingliang, ZHENG Zhenhuan, OUYANG Sheng, HE Wen, CHEN Weihua, LIU Changhong, ZHANG Jianjun, LIU Zhiyong. Facile Synthesis and Supercapacitor Performance of M3O4(M=FeCoCrMnMg) High Entropy Oxide Powders [J]. Journal of Inorganic Materials, 2021, 36(4): 425-430. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||