
Journal of Inorganic Materials ›› 2021, Vol. 36 ›› Issue (8): 856-864.DOI: 10.15541/jim20200663
Special Issue: 【虚拟专辑】放射性污染物去除(2020~2021); 【能源环境】水体污染物去除
• RESEARCH ARTICLE • Previous Articles Next Articles
YU Xiangkun1( ), LIU Kun1, LI Zhipeng1, ZHAO Yulu1, SHEN Jinyou2, MAO Ping1(
), LIU Kun1, LI Zhipeng1, ZHAO Yulu1, SHEN Jinyou2, MAO Ping1( ), SUN Aiwu1(
), SUN Aiwu1( ), JIANG Jinlong1
), JIANG Jinlong1
Received:2020-11-19
Revised:2021-01-04
Published:2021-08-20
Online:2021-03-01
Contact:
MAO Ping, associate professor. E-mail: pingmao@hyit.edu.cn; SUN Aiwu, professor. E-mail: sunaiwu@hyit.edu.cn
About author:YU Xiangkun(1993-), male, Master candidate. E-mail: yxk0079@163.com
Supported by:CLC Number:
YU Xiangkun, LIU Kun, LI Zhipeng, ZHAO Yulu, SHEN Jinyou, MAO Ping, SUN Aiwu, JIANG Jinlong. Efficient Adsorption of Radioactive Iodide by Copper/Palygorskite Composite[J]. Journal of Inorganic Materials, 2021, 36(8): 856-864.
| Composition | PAL | Cu@PAL |
|---|---|---|
| SiO2 | 60.96 | 59.87 |
| MgO | 9.36 | 8.12 |
| Al2O3 | 12.20 | 11.07 |
| Fe2O3 | 8.00 | 7.51 |
| CaO | 5.55 | 0.46 |
| CuO | 0.01 | 9.83 |
| Na2O | 0.12 | 0.01 |
| LOI | 3.80 | 3.13 |
Table1 Chemical analysis (XRF) of PAL and Cu@PAL/wt%
| Composition | PAL | Cu@PAL |
|---|---|---|
| SiO2 | 60.96 | 59.87 |
| MgO | 9.36 | 8.12 |
| Al2O3 | 12.20 | 11.07 |
| Fe2O3 | 8.00 | 7.51 |
| CaO | 5.55 | 0.46 |
| CuO | 0.01 | 9.83 |
| Na2O | 0.12 | 0.01 |
| LOI | 3.80 | 3.13 |
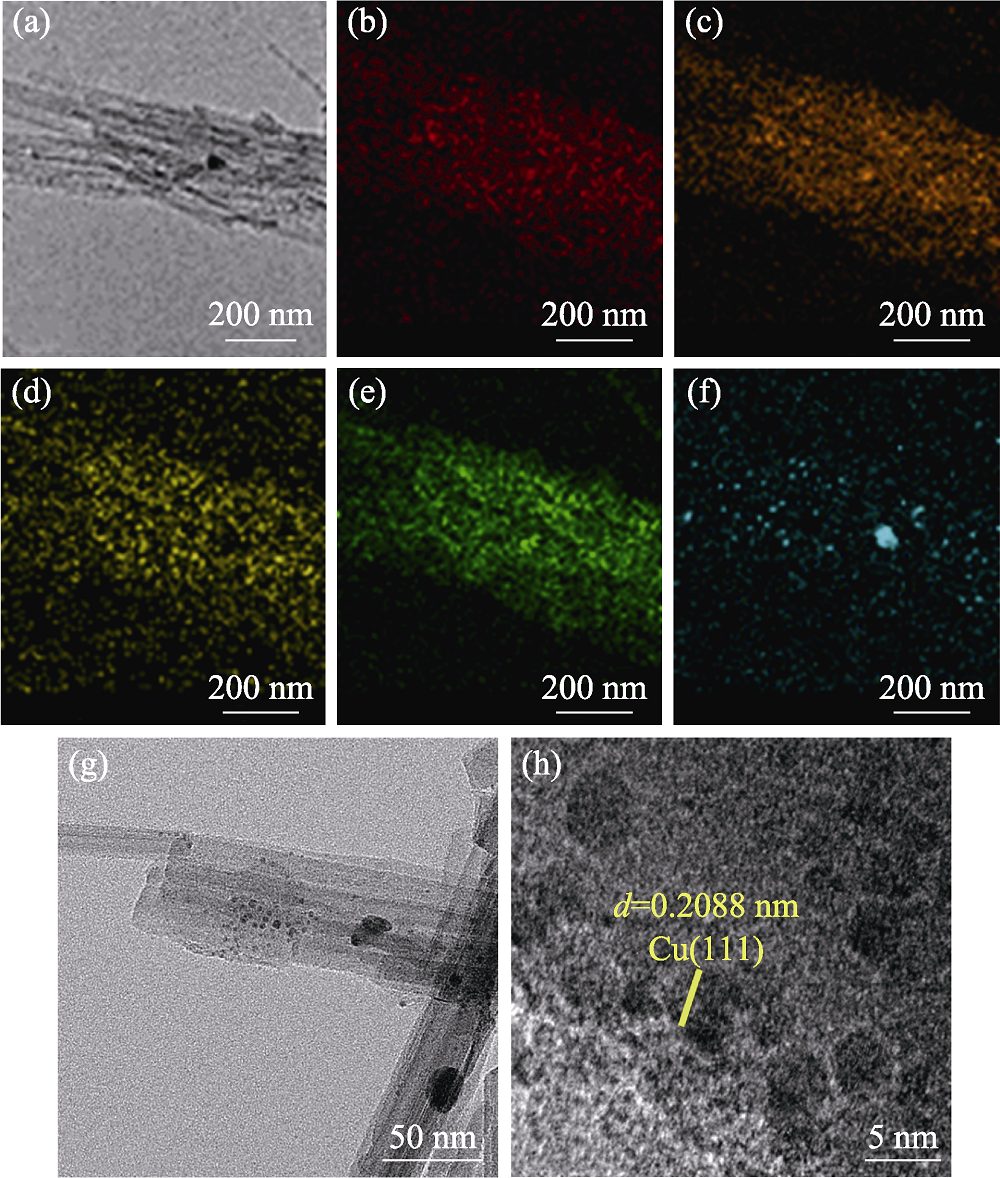
Fig. 4 (a) TEM image of Cu@PAL and elemental mappings of (b) N, (c) O, (d) Al, (e) Si, and (f) Cu; and high magnification TEM (g) and HRTEM (h) images of Cu@PAL
| Adsorbent | pH | Qe/ (mg∙g-1) | Utilization efficiency/% | Ref. |
|---|---|---|---|---|
| Cuprite sulfide | 7 | 6.1 | — | [ |
| Cu2O/Cu-C | 7 | 41.2 | 10.38 | [ |
| Hollow Cu/Cu2O | 7 | 33.0 | 1.85 | [ |
| Core-shell Cu/Cu2O | 7 | 22.9 | 1.4 | [ |
| Cu | 7 | 6.35 | 0.32 | [ |
| Cu/PAL | 7 | 116.1 | 71.98 | This work |
Table 2 Comparison of several Cu based adsorbents for iodide adsorption
| Adsorbent | pH | Qe/ (mg∙g-1) | Utilization efficiency/% | Ref. |
|---|---|---|---|---|
| Cuprite sulfide | 7 | 6.1 | — | [ |
| Cu2O/Cu-C | 7 | 41.2 | 10.38 | [ |
| Hollow Cu/Cu2O | 7 | 33.0 | 1.85 | [ |
| Core-shell Cu/Cu2O | 7 | 22.9 | 1.4 | [ |
| Cu | 7 | 6.35 | 0.32 | [ |
| Cu/PAL | 7 | 116.1 | 71.98 | This work |
| Langmuir model | Frenudlich model | ||||
|---|---|---|---|---|---|
| Qm/(mg∙g-1) | Kl | R2 | Kf | 1/n | R2 |
| 1.02915 | 0.86041 | 0.99632 | 0.38173 | 0.51042 | 0.81026 |
Table 3 Isotherm parameters for the adsorption of I- anions by Cu@PAL
| Langmuir model | Frenudlich model | ||||
|---|---|---|---|---|---|
| Qm/(mg∙g-1) | Kl | R2 | Kf | 1/n | R2 |
| 1.02915 | 0.86041 | 0.99632 | 0.38173 | 0.51042 | 0.81026 |
| Time/h | Qe/(mg∙g-1) | |
|---|---|---|
| Cu@PAL | Nano-Cu | |
| 0 | 74.2 | 234.7 |
| 12 | 69.4 | 133.8 |
| 24 | 68.1 | 110.4 |
| 48 | 64.1 | 104.6 |
| 72 | 60.2 | 96.3 |
| 144 | 58.7 | 88.4 |
Table 4 Adsorption properties of Cu@PAL and nano Cu exposed to air
| Time/h | Qe/(mg∙g-1) | |
|---|---|---|
| Cu@PAL | Nano-Cu | |
| 0 | 74.2 | 234.7 |
| 12 | 69.4 | 133.8 |
| 24 | 68.1 | 110.4 |
| 48 | 64.1 | 104.6 |
| 72 | 60.2 | 96.3 |
| 144 | 58.7 | 88.4 |
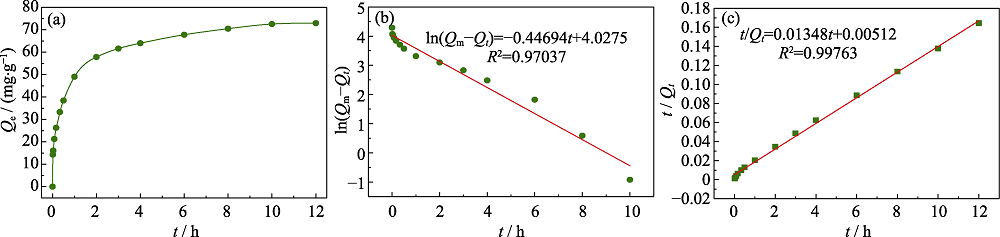
Fig. 11 Absorption kinetic curve (a), fitting curves of the pseudo first order kinetic model (b) and pseudo second order kinetic model (c) for adsorption kinetic of Cu@PAL
| Pseudo-first-order | Pseudo-second-order | ||||
|---|---|---|---|---|---|
| Qm/ (mg∙g-1) | k1/(g∙(mmol∙ g-1)-1) | R2 | Qm/ (mg∙g-1) | k2/(g∙(mmol∙ g-1)-1) | R2 |
| 56.10 | 0.4469 | 0.9704 | 74.1840 | 0.03549 | 0.9976 |
Table 5 Kinetic parameters for the adsorption of I- by Cu@PAL
| Pseudo-first-order | Pseudo-second-order | ||||
|---|---|---|---|---|---|
| Qm/ (mg∙g-1) | k1/(g∙(mmol∙ g-1)-1) | R2 | Qm/ (mg∙g-1) | k2/(g∙(mmol∙ g-1)-1) | R2 |
| 56.10 | 0.4469 | 0.9704 | 74.1840 | 0.03549 | 0.9976 |
| Adsorbent | cNaCl/(mol∙L-1) | Desorption efficiency/% |
|---|---|---|
| Cu@PAL | 0 | 21.3 |
| 0.1 | 32.1 | |
| Nano-Cu | 0 | 87.4 |
| 0.1 | 94.1 |
Table 6 Leaching or desorption efficiencies of Cu@PAL and nano-Cu after adsorption
| Adsorbent | cNaCl/(mol∙L-1) | Desorption efficiency/% |
|---|---|---|
| Cu@PAL | 0 | 21.3 |
| 0.1 | 32.1 | |
| Nano-Cu | 0 | 87.4 |
| 0.1 | 94.1 |
| [1] | KBERGER T. Progress of renewable electricity replacing fossil fuels. Global Energy Interconnection , 2018, 1(1):48-52. |
| [2] |
TRUESDALE V W, NAUSCH G, BAKER A. The distribution of iodine in the Baltic Sea during summer. Mar. Chem. , 2001, 74(2):87-98.
DOI URL |
| [3] |
GMEZ-GUZM N J M, HOLM E, NIAGOLOVA N, et al. Influence of releases of 129I and 137Cs from European reprocessing facilities in Fucus vesiculosus and seawater from the Kattegat and Skagerrak areas. Chemosphere , 2014, 108:76-84.
DOI URL |
| [4] |
LI C, WEI Y, WANG X, et al. Efficient and rapid adsorption of iodide ion from aqueous solution by porous silica spheres loaded with calcined Mg-Al layered double hydroxide. Journal of the Taiwan Institute of Chemical Engineers , 2018, 85:193-200.
DOI URL |
| [5] |
HOSKINS J S, KARANFIL T, SERKIZ S M. Removal and sequestration of iodide using silver-impregnated activated carbon. Environ. Sci. Technol. , 2002, 36(4):784-789.
DOI URL |
| [6] |
THEISS F L, AYOKO G A, FROST R L. Iodide removal using LDH technology. Chemical Engineering Journal , 2016, 296:300-309.
DOI URL |
| [7] |
INOUE H. Effects of Co-ions on transport of iodide ions through a non-conventional anion exchange paper membrane. J. Membr. Sci. , 2004, 228(2):209-215.
DOI URL |
| [8] |
LIU S, WANG N, ZHANG Y, et al. Efficient removal of radioactive iodide ions from water by three-dimensional Ag2O-Ag/TiO2 composites under visible light irradiation. J. Hazard Mater. , 2015, 284:171-181.
DOI URL |
| [9] |
MAO P, LIU Y, JIAO Y, et al. Enhanced uptake of iodide on Ag@Cu2O nanoparticles. Chemosphere , 2016, 164:396-403.
DOI URL |
| [10] |
CHOI M H, JEONG S W, SHIM H E, et al. Efficient bioremediation of radioactive iodine using biogenic gold nanomaterial-containing radiation-resistant bacterium, Deinococcus radiodurans R1. Chemical Communications , 2017, 53(28):3937-3940.
DOI URL |
| [11] |
ZHANG W, LI Q, MAO Q, et al. Cross-linked chitosan microspheres: an efficient and eco-friendly adsorbent for iodide removal from waste water. Carbohydr. Polym. , 2019, 209:215-222.
DOI URL |
| [12] |
MAO P, QI L, LIU X, et al. Synthesis of Cu/Cu2O hydrides for enhanced removal of iodide from water. J. Hazard Mater. , 2017, 328:21-28.
DOI URL |
| [13] | LIU L, LIU W, ZHAO X, et al. Selective capture of iodide from solutions by microrosette-like δ-Bi2O3. ACS Applied Materials & Interfaces , 2014, 6(18):16082-16090. |
| [14] |
MAILEN J C, HORNER D E. Removal of iodine from reactor fuel solutions as insoluble PdI2. Nucl. Technol. , 1977, 33(3):260-263.
DOI URL |
| [15] |
BALSLEY S D, BRADY P V, KRUMHANSL J L, et al. Iodide retention by metal sulfide surfaces: cinnabar and chalcocite. Environ. Sci. Technol. , 1996, 30(10):3025-3027.
DOI URL |
| [16] | YAQUAN W, FENG Y, JIANG J, et al. Designing of recyclable attapulgite for wastewater treatments: a review. ACS Sustainable Chemistry & Engineering , 2018, 7(2):1855-1869. |
| [17] |
CHEN H, ZHAO Y, WANG A. Removal of Cu(II) from aqueous solution by adsorption onto acid-activated palygorskite. J. Hazard Mater. , 2007, 149(2):346-354.
DOI URL |
| [18] | 王爱勤, 王文波, 郑易安, et al. 凹凸棒石棒晶束解离及其纳米化功能复合材料. 北京: 科学出版社, 2014. |
| [19] | ZHOU Y, HE J, WANG H, et al. Carbon nanofiber yarns fabricated from co-electrospun nanofibers. Materials & Design , 2016, 95:591-598. |
| [20] |
MAO P, JIANG J, PAN Y, et al. Enhanced uptake of iodide from solutions by hollow Cu-based adsorbents. Materials , 2018, 11(5):769.
DOI URL |
| [21] |
MAO P, YU X, LIU K, et al. Rapid and reversible adsorption of radioactive iodide from wastewaters by green and low-cost palygorskite-based microspheres. J. Radioanal Nucl. Chem. , 2020, 325(1):303-313.
DOI URL |
| [22] |
ZHANG Y, YU C, HU P, et al. Mechanical and thermal properties of palygorskite poly(butylene succinate) nanocomposite. Applied Clay Science , 2016, 119:96-102.
DOI URL |
| [23] |
CARAZO E, BORREGO-S NCHEZ A, GARC A-VILL N F, et al. Adsorption and characterization of palygorskite-isoniazid nanohybrids. Applied Clay Science , 2018, 160:180-185.
DOI URL |
| [24] |
BA N, ZHU L, LI H, et al. 3D rod-like copper oxide with nanowire hierarchical structure: ultrasound assisted synthesis from Cu2(OH)3NO3 precursor, optical properties and formation mechanism. Solid State Sciences , 2016, 53:23-29.
DOI URL |
| [25] |
YAO S, ZHANG H, CHEN Z, et al. Promotion of graphitic carbon oxidation via stimulating CO2 desorption by calcium carbonate. J. Hazard Mater. , 2019, 363:10-15.
DOI URL |
| [26] |
THOMMES M, KANEKO K, NEIMARK A V, et al. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. , 2015, 87(9/10):1051-1069.
DOI URL |
| [27] |
LEF VRE G, BESSI RE J, EHRHARDT J J, et al. Immobilization of iodide on copper(I) sulfide minerals. Journal of Environmental Radioactivity , 2003, 70(1/2):73-83.
DOI URL |
| [28] |
ZHANG X, GU P, LI X, et al. Efficient adsorption of radioactive iodide ion from simulated wastewater by nano Cu2O/Cu modified activated carbon. Chemical Engineering Journal , 2017, 322:129-139.
DOI URL |
| [29] |
HAQ Z, BANCROFT G M, FYFE W S, et al. Sorption of iodide on copper. Environ. Sci. Technol. , 1980, 14(9):1106-1110.
DOI URL |
| [30] |
WU C K, YIN M, O'BRIEN S, et al. Quantitative analysis of copper oxide nanoparticle composition and structure by X-ray photoelectron spectroscopy. Chemistry of Materials , 2006, 18(25):6054-6058.
DOI URL |
| [1] | WANG Wei-Qing,FENG Qi-Ming,DONG Fa-Qin,LI Hu-Jie,ZHAO Xiao-Dong. Preparation and Properties of Fe3O4/Clinoptilolite Magnetic Composite [J]. Journal of Inorganic Materials, 2010, 25(4): 401-405. |
| [2] | LI Zhi-Liang,ZHI Jian-Ping,ZHANG Yu-Lin. Effects of Different Starting Materials on Preparation and Adsorption Properties of the Mixed Li+ and Ca2+ Exchanged Low Silica X-type Zeolites(LSX) [J]. Journal of Inorganic Materials, 2008, 23(5): 975-980. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||