
Journal of Inorganic Materials ›› 2021, Vol. 36 ›› Issue (6): 623-628.DOI: 10.15541/jim20200534
Special Issue: 能源材料论文精选(2021); 【虚拟专辑】计算材料
• RESEARCH ARTICLE • Previous Articles Next Articles
ZHANG Xiaojun1( ), LI Jiale1,2, QIU Wujie2,3, YANG Miaosen1, LIU Jianjun2,3,4(
), LI Jiale1,2, QIU Wujie2,3, YANG Miaosen1, LIU Jianjun2,3,4( )
)
Received:2020-09-14
Revised:2020-11-13
Published:2021-06-20
Online:2020-12-01
Contact:
LIU Jianjun, professor. E-mail: jliu@mail.sic.ac.cn
About author:ZHANG Xiaojun(1974-), female, senior experimentalist. E-mail: zhangxjun1123@126.com
Supported by:CLC Number:
ZHANG Xiaojun, LI Jiale, QIU Wujie, YANG Miaosen, LIU Jianjun. Electrochemical Activity of Positive Electrode Material of P2-Nax[Mg0.33Mn0.67]O2 Sodium Ion Battery[J]. Journal of Inorganic Materials, 2021, 36(6): 623-628.
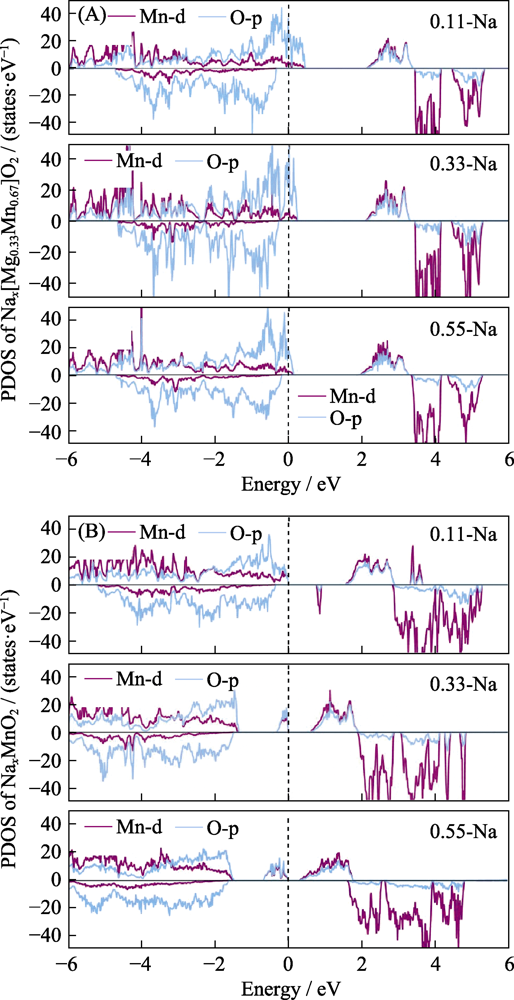
Fig. 5 Electronic density of states of (A) P2-Nax[Mg0.33Mn0.67]O2 and (B) P2-NaxMnO2 under different Na ion contents during discharge PDOS: projected density of states
| [1] | HU YING-YING, WEN ZHAO-YIN, RUI-KUN, et al. State-of- the-art research and development status of sodium batteries. Energy Storage Science and Technology, 2013,2(2):81-90. |
| [2] | SHEN GUAN-YE, LI CHEN, XU BING-LIANG, et al. Economic allocation for energy storage system considering wind power. Journal of Northeast Electric Power University, 2018,38(4):27-34. |
| [3] |
MA CHAO, ZHAO XIAO-LIN, KANG LI-TAO, et al. Non- conjugated dicarboxylate anode materials for electrochemical cells. Angew. Chem. Int. Ed., 2018,57(29):8865-8870.
DOI URL |
| [4] |
RICHARDS W D, DACEK S T, KITCHAEV D A, et al. Fluorination of lithium-excess transition metal oxide cathode materials. Advanced Energy Materials, 2018,8(5):1701533.
DOI URL |
| [5] |
XIANG XING-DE, ZHANG KAI, CHEN JUN. Recent advances and prospects ofcathode materials for sodium-ion batteries. Adv. Mater., 2015,27(36):5343-5364.
DOI URL |
| [6] | MA CHAO, ZHAO XIAO-LIN, HARRIS M M, et al. Uric acid as an electrochemically active compound for sodium-ion batteries: stepwise Na+-storage mechanisms of π-conjugation and stabilized carbon anion. ACS Applied Materials & Interfaces, 2017,9(39):33934-33940. |
| [7] |
LEE D H, XU JING, MENG Y S. An advanced cathode for Na-ion batteries with high rate and excellent structural stability. Phys. Chem. Chem. Phys., 2013,15(9):3304-3312.
DOI URL |
| [8] |
KUBOTA K, YABUUCHI N, YOSHIDA H, et al. Layered oxides as positive electrode materials for Na-ion batteries. MRS Bulletin, 2014,39(5):416-422.
DOI URL |
| [9] |
CLÉMENT R J, BRUCE P G, GREY C P. Review—manganese- based P2-type transition metal oxides as sodium-ionbattery cathode materials. Journal of the Electrochemical Society, 2015,162(14):A2589-A2604.
DOI URL |
| [10] |
BERTHELOT R, CARLIER D, DELMAS C. Electrochemical investigation of the P2-NaxCoO2 phase diagram. Nat. Mater., 2011,10(1):74-80.
DOI URL |
| [11] |
YABUUCHI N, HARA R, KUBOTA K, et al. A new electrode material for rechargeable sodium batteries: P2-type Na2/3[Mg0.28Mn0.72]O2 with anomalously high reversible capacity. J. Mater. Chem. A, 2014,2(40):16851-16855.
DOI URL |
| [12] |
MAITRA U, HOUSE R A, SOMERVILLE J W, et al. Oxygen redox chemistry without excess alkali-metal ions in Na2/3[Mg0.28Mn0.72]O2. Nat. Chem., 2018,10(3):288-295.
DOI URL |
| [13] |
GUO SHAO-HUA, SUN YANG, YI JIN, et al. Understanding sodium-ion diffusion in layered P2 and P3 oxides via experiments and first-principles calculations: a bridge between crystal structure and electrochemical performance. NPG Asia Materials, 2016,8:e266.
DOI URL |
| [14] |
JI HUI-WEI, KITCHAEV D A, LUN ZHANG-YAN, et al. Computational investigation and experimental realization of disordered high-capacity Li-ion cathodes based on Ni redox. Chemistry of Materials, 2019,31(7):2431-2442.
DOI URL |
| [15] |
LEE J, URBAN A, LI XIN, et al. Unlocking the potential of cation-disordered oxides for rechargeable lithium batteries. Science, 2014,343(6170):519-522.
DOI URL |
| [16] |
URBAN A, LEE J, CEDER G. The configurational space of rocksalt-type oxides for high-capacity lithium battery electrodes. Advanced Energy Materials, 2014,4(13):1400478.
DOI URL |
| [17] |
CHAKRABORTY A, DIXIT M, AURBACH D, et al. Predicting accurate cathode properties of layered oxide materials using the SCAN meta-GGA density functional. npj Computational Materials, 2018,4:60.
DOI URL |
| [18] |
URBAN A, ABDELLAHI A, DACEK S, et al. Electronic-structure origin of cation disorder in transition-metal oxides. Phys. Rev. Lett., 2017,119(17):176402.
DOI URL |
| [19] |
ASSAT G, TARASCON J M. Fundamental understanding and practical challenges of anionic redox activity in Li-ion batteries. Nature Energy, 2018,3(5):373-386.
DOI URL |
| [20] |
YABUUCHI N, NAKAYAMA M, TAKEUCHI M, et al. Origin of stabilization and destabilization in solid-state redox reaction of oxide ions for lithium-ion batteries. Nat. Commun., 2016,7:13814.
DOI URL |
| [21] |
SANNYAL A, AHN Y, JANG J. First-principles study on the two-dimensional siligene (2D SiGe) as an anode material of an alkali metal ion battery. Computational Materials Science, 2019,165:121-128.
DOI URL |
| [22] |
LI HONG, HU YONG-SHENG, PAN HUI-LIN, et al. Research progress on electrode material structure of room temperature sodium ion storage battery. Scientia Sinica Chimica, 2014,44(8):1269-1279.
DOI URL |
| [23] |
WANG YUE-SHENG, XIAO RUI-JUAN, HU YONG-SHENG, et al. P2-Na0.6[Cr0.6Ti0.4]O2 cation-disordered electrode for high-rate symmetric rechargeable sodium-ion batteries. Nat. Commun., 2015,6:6954.
DOI URL |
| [24] |
WANG QIN-CHAO, MENG JING-KE, YUE XIN-YANG, et al. Tuning P2-structured cathode material by Na-site Mg substitution for Na-ion batteries. J. Am. Chem. Soc., 2019,141(2):840-848.
DOI URL |
| [25] | MENDIBOURD A, DELMAS C, HAGENMULLER C. Electrochemical intercalation and deintercalation of NaxMnO2 bronzes. Academic Press, 1985,57(3):323-331. |
| [26] | SOMERVILLE J W, SOBKOWIAK A, TAPIA-RUIZ N, et al. Nature of the “Z”-phase in layered Na-ion battery cathodes. Energy & Environmental Science, 2019,12(7):2223-2232. |
| [27] |
QU JIE, WANG DONG, YANG ZU-GUANG, et al. Ion-doping- site-variation-induced composite cathode adjustment: a case study of layer-tunnel Na0.6MnO2 with Mg2+ doping at Na/Mn site. ACS Appl. Mater. Interfaces, 2019,11(30):26938-26945.
DOI URL |
| [28] |
SATO T, SATO K, ZHAO WEN-WEN, et al. Metastable and nanosize cation-disordered rocksalt-type oxides: revisit of stoichiometric LiMnO2 and NaMnO2. Journal of Materials Chemistry A, 2018,6(28):13943-13951.
DOI URL |
| [29] | GUIGNARD M, DELMAS C. Using a battery to synthesize new vanadium oxides. Chemistry Select, 2017,2(20):5800-5804. |
| [30] | WANG PENG-FEI, YAO HU-RONG, LIU XIN-YU, , et al. Na+/ vacancy disordering promises high-rate Na-ion batteries. Science Advances, 2018, 4(3): eaar6018. |
| [31] |
KIM H, KIM D J, SEO D H, et al. Ab initio study of the sodium intercalation and intermediate phases in Na0.44MnO2 for sodium-ion battery. Chemistry of Materials, 2012,24(6):1205-1211.
DOI URL |
| [32] |
LI XIN, MA XIAO-HUA, SU DONG, et al. Direct visualization of the Jahn-Teller effect coupled to Na ordering in Na5/8MnO2. Nat. Mater., 2014,13(6):586-592.
DOI URL |
| [33] | WANG YOUWEI, WANG JUNKAI, ZHAO XIAOLIN, et al. Reducing the charge overpotential of Li-O2 batteries through band-alignment cathode design. Energy & Environmental Science, 2020,13(8):2540-2548. |
| [34] |
ZHENG C, RADHAKRISHNAN B, CHU I H, et al. Effects of transition-metal mixing on Na ordering and kinetics inlayered P2 oxides. Physical Review Applied, 2017,7(6):064003.
DOI URL |
| [35] |
LUN ZHENG-YAN, OUYANG B, CAI ZI-JIAN, et al. Design principles for high-capacity Mn-based cation-disordered rocksalt cathodes. Chem, 2020,6(1):153-168.
DOI URL |
| [36] |
SEO D H, LEE J, URBAN A, et al. The structural and chemical origin of the oxygen redox activity in layered and cation-disordered Li-excess cathode materials. Nat. Chem., 2016,8(7):692-697.
DOI URL |
| [37] |
BAI QIANG, YANG LU-FENG, CHEN HAI-LONG, et al. Computational studies of electrode materials in sodium-ion batteries. Advanced Energy Materials, 2018,8(17):1702998.
DOI URL |
| [1] | ZHANG Shouchao, CHEN Hongyu, LIU Hongfei, YANG Yu, LI Xin, LIU Defeng. High Temperature Recovery of Neutron Irradiation-induced Swelling and Optical Property of 6H-SiC [J]. Journal of Inorganic Materials, 2023, 38(6): 678-686. |
| [2] | YANG Yingkang, SHAO Yiqing, LI Bailiang, LÜ Zhiwei, WANG Lulu, WANG Liangjun, CAO Xun, WU Yuning, HUANG Rong, YANG Chang. Enhanced Band-edge Luminescence of CuI Thin Film by Cl-doping [J]. Journal of Inorganic Materials, 2023, 38(6): 687-692. |
| [3] | SUN Ming, SHAO Puzhen, SUN Kai, HUANG Jianhua, ZHANG Qiang, XIU Ziyang, XIAO Haiying, WU Gaohui. First-principles Study on Interface of Reduced Graphene Oxide Reinforced Aluminum Matrix Composites [J]. Journal of Inorganic Materials, 2022, 37(6): 651-659. |
| [4] | FENG Qingying, LIU Dong, ZHANG Ying, FENG Hao, LI Qiang. Thermodynamic and First-principles Assessments of Materials for Solar-driven CO2 Splitting Using Two-step Thermochemical Cycles [J]. Journal of Inorganic Materials, 2022, 37(2): 223-229. |
| [5] | ZHAO Wei, XU Yang, WAN Yingjie, CAI Tianxun, MU Jinxiao, HUANG Fuqiang. Metal Cyanamides/Carbodiimides: Structure, Synthesis and Electrochemical Energy Storage Performance [J]. Journal of Inorganic Materials, 2022, 37(2): 140-151. |
| [6] | ZENG Fanxin, LIU Chuang, CAO Yuliang. Sodium Storage Behavior of Nanoporous Sb/MCNT Anode Material with High Cycle Stability by Dealloying Route [J]. Journal of Inorganic Materials, 2021, 36(11): 1137-1144. |
| [7] | ZHAO Yupeng,HE Yong,ZHANG Min,SHI Junjie. First-principles Study on the Photocatalytic Hydrogen Production of a Novel Two-dimensional Zr2CO2/InS Heterostructure [J]. Journal of Inorganic Materials, 2020, 35(9): 993-998. |
| [8] | LI Jin, LIU Ting-Yu, YAO Shu-An, FU Ming-Xue, LU Xiao-Xiao. First Principles Study on the Property of O Vacancy in LuPO4 Crystal [J]. Journal of Inorganic Materials, 2019, 34(8): 879-884. |
| [9] | LI Xin, XI Li-Li, YANG Jiong. First Principles High-throughput Research on Thermoelectric Materials: a Review [J]. Journal of Inorganic Materials, 2019, 34(3): 236-246. |
| [10] | ZOU Ai-Hua, ZHOU Xian-Liang, KANG Zhi-Bing, RAO You-Hai, WU Kai-Yang. Alloy Elements on SiC/Al Interface: a First-principle and Experimental Study [J]. Journal of Inorganic Materials, 2019, 34(11): 1167-1174. |
| [11] | WANG Zhong, ZHA Xian-Hu, WU Ze, HUANG Qing, DU Shi-Yu. First-principles Study on Electronic and Magnetic Properties of Mn-doped Strontium Ferrite SrFe12O19 [J]. Journal of Inorganic Materials, 2019, 34(10): 1047-1054. |
| [12] | ZHAO Hai-Bing, XU Hai-Feng, YANG Ke-Wei, LIN Chen-Xue, FENG Miao, YU Yan. Enhanced Photoreversible Color Switching of Methylene Blue Catalyzed by Magnesium-doped TiO2 Nanocrystals [J]. Journal of Inorganic Materials, 2018, 33(10): 1124-1130. |
| [13] | WANG Shu-Wei, HU He-Feng, WANG De-Yu, SHEN Cai. AFM Investigation of Solid Electrolyte Interphase on HOPG Anode in Sodium Ion Battery [J]. Journal of Inorganic Materials, 2017, 32(6): 596-602. |
| [14] | CHANG Xi-Wang, CHEN Ning, WANG Li-Jun, LI Fu-Shen, BIAN Liu-Zhen, CHOU Kuo-Chih. Optimal Principle on Composition of B Site Elements in Perovskite Electrodes with Sr at A Site for Solid Oxide Fuel Cell [J]. Journal of Inorganic Materials, 2017, 32(10): 1055-1062. |
| [15] | CHANG Xi-Wang, CHEN Ning, WANG Li-Jun, BIAN Liu-Zhen, LI Fu-Shen, CHOU Kuo-Chih. Optimization Rule of Anode Materials for Solid Oxide Fuel Cells [J]. Journal of Inorganic Materials, 2015, 30(10): 1043-1048. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||