
Journal of Inorganic Materials ›› 2021, Vol. 36 ›› Issue (4): 355-364.DOI: 10.15541/jim20200366
Special Issue: 【结构材料】超高温结构陶瓷; 【结构材料】高熵陶瓷
Previous Articles Next Articles
WANG Haoxuan1( ), LIU Qiaomu2, WANG Yiguang3(
), LIU Qiaomu2, WANG Yiguang3( )
)
Received:2020-07-02
Revised:2020-09-27
Published:2021-04-20
Online:2020-09-20
Contact:
WANG Yiguang, professor. E-mail: wangyiguang@bit.edu.cn
About author:WANG Haoxuan(1994-), male, PhD candidate. E-mail: wanghaoxuan@mail.nwpu.edu.cn
Supported by:CLC Number:
WANG Haoxuan, LIU Qiaomu, WANG Yiguang. Research Progress of High Entropy Transition Metal Carbide Ceramics[J]. Journal of Inorganic Materials, 2021, 36(4): 355-364.
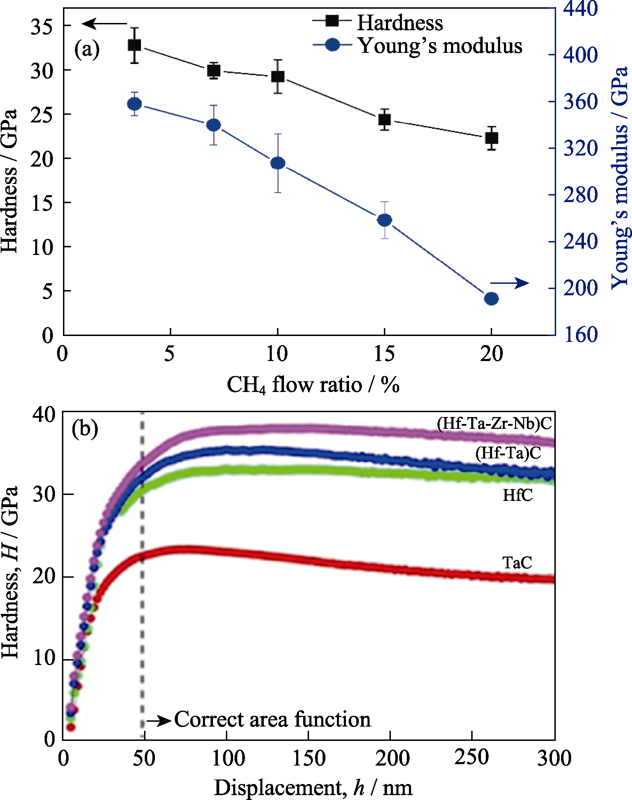
Fig. 2 (a) Hardness of the CrNbSiTiZrCx with different carbon contents[84], and (b) hardness depth-profles of the individual, binary and high-entropy carbide[87]
| HEC | Hardness/GPa | Average/GPa |
|---|---|---|
| HfC[ | 25 | — |
| TaC[ | 14 | — |
| ZrC[ | 24 | — |
| TiC[ | 31 | — |
| NbC[ | 17 | — |
| WC[ | 14 | — |
| VC[ | 29 | — |
| Mo2C[ | 27 | — |
| (ZrNbTiV)C[ | 30 | 25 |
| (HfTaZrNb)C[ | 36 | 20 |
| (TiVNbTaW)C[ | 28 | 21 |
| (TiHfTaWZr)C[ | 33 | 22 |
| (TiHfNbTaMo)C[ | 27 | 23 |
| (TiZrNbTaMo)C[ | 32 | 23 |
| (VNbTaMoW)C[ | 27 | 20 |
| (HfTaZrTiNb)C[ | 32 | 28 |
| (TiZrHfTaW)C[ | 24 | 22 |
| (TiHfNbTaW)C[ | 31 | 20 |
| (TiHfVNbTa)C[ | 29 | 23 |
Table 1 Hardness of some carbide ceramics[31,74,88-90]
| HEC | Hardness/GPa | Average/GPa |
|---|---|---|
| HfC[ | 25 | — |
| TaC[ | 14 | — |
| ZrC[ | 24 | — |
| TiC[ | 31 | — |
| NbC[ | 17 | — |
| WC[ | 14 | — |
| VC[ | 29 | — |
| Mo2C[ | 27 | — |
| (ZrNbTiV)C[ | 30 | 25 |
| (HfTaZrNb)C[ | 36 | 20 |
| (TiVNbTaW)C[ | 28 | 21 |
| (TiHfTaWZr)C[ | 33 | 22 |
| (TiHfNbTaMo)C[ | 27 | 23 |
| (TiZrNbTaMo)C[ | 32 | 23 |
| (VNbTaMoW)C[ | 27 | 20 |
| (HfTaZrTiNb)C[ | 32 | 28 |
| (TiZrHfTaW)C[ | 24 | 22 |
| (TiHfNbTaW)C[ | 31 | 20 |
| (TiHfVNbTa)C[ | 29 | 23 |
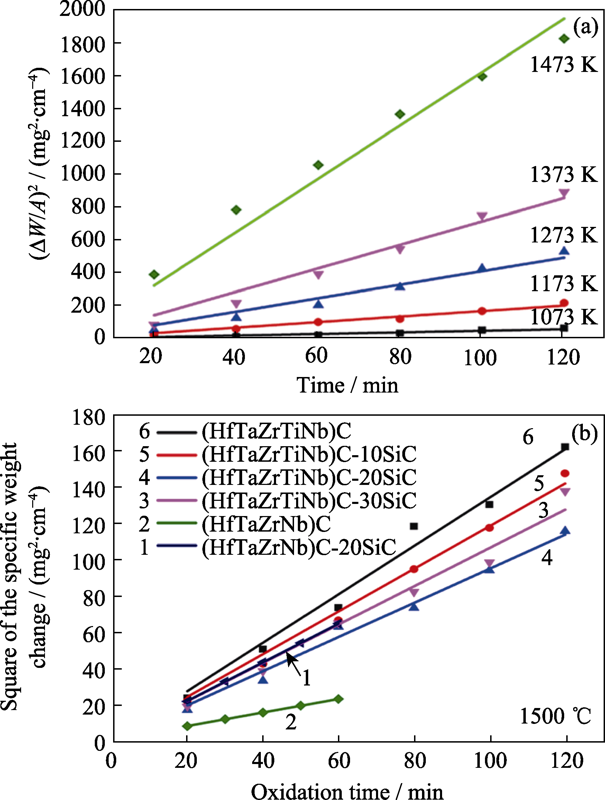
Fig. 4 (a) Square of the specific weight change as a function of oxidation time at 800-1200 ℃ for (Hf0.2Zr0.2Ta0.2Nb0.2Ti0.2)C[95], (b) square of the specific weight change as a function of oxidation time at 1500 ℃ for high-entropy carbide in different systems[33,97,99]
| [1] | MIRACLE D B, SENKOV O N. A critical review of high entropy alloys and related concepts. Acta Mater., 2017,122:448-511. |
| [2] | YEH J W, CHEN S K, LIN S J, et al. Nanostructured high- entropy alloys with multiple principal elements: novel alloy design concepts and outcomes. Adv. Eng. Mater., 2004,6(5):299-303. |
| [3] |
KUCZA W, DABROWA J, CIESLAK G, et al. Studies of “sluggish diffusion” effect in Co-Cr-Fe-Mn-Ni, Co-Cr-Fe-Ni and Co-Fe-Mn-Ni high entropy alloys; determination of tracer diffusivities by combinatorial approach. J. Alloys Compd., 2018,731:920-928.
DOI URL |
| [4] | UTT D, STUKOWSKI A, ALBE K. Grain boundary structure and mobility in high-entropy alloys: a comparative molecular dynamics study on a 11 symmetrical tilt grain boundary in face-centered cubic CuNiCoFe. Acta Mater., 2020,186:11-19. |
| [5] |
GLUDOVATZ B, HOHENWARTER A, CATOOR D, et al. A fracture-resistant high-entropy alloy for cryogenic applications. Science, 2014,345(6201):1153-1158.
URL PMID |
| [6] |
LI Z M, PRADEEP K G, DENG Y, et al. Metastable high-entropy dual-phase alloys overcome the strength-ductility trade-off. Nature, 2016,534(7606):227-230.
URL PMID |
| [7] | ZHANG R Z, REECE M J. Review of high entropy ceramics: design, synthesis, structure and properties. J. Mater. Chem. A, 2019,7(39):22148-22162. |
| [8] | MURTY B S, YEH J W, RANGANATHAN S, et al. High-entropy Alloys. United Kingdom: Butterworth-Heinemann, 2019: 165-176. |
| [9] | OSES C, TOHER C, CURTAROLO S. High-entropy ceramics. Nat. Rev. Mater., 2020,5(4):295-309. |
| [10] | LAL M S, SUNDARA R. High entropy oxides-a cost-effective catalyst for the growth of high yield carbon nanotubes and their energy applications. ACS Appl. Mater. Inter., 2019,11(34):30846-30857. |
| [11] |
SARKAR A, VELASCO L, WANG D, et al. High entropy oxides for reversible energy storage. Nat. Commun., 2018,9(1):3400.
URL PMID |
| [12] | BERARDAN D, FRANGER S, DRAGOE D, et al. Colossal dielectric constant in high entropy oxides. Phys. Status Solidi-R, 2016,10(4):328-333. |
| [13] | BIESUZ M, SPIRIDIGLIOZZI L, DELLAGLI G, et al. Synthesis and sintering of (Mg,Co,Ni,Cu,Zn)O entropy-stabilized oxides obtained by wet chemical methods. J. Mater. Sci., 2018,53(11):8074-8085. |
| [14] |
OKEJIRI F, ZHANG Z H, LIU J X, et al. Room-temperature synthesis of high-entropy perovskite oxide nanoparticle catalysts via ultrasonication-based method. ChemSusChem, 2020,13(1):111-115.
DOI URL PMID |
| [15] | DEMIRSKYI D, BORODIANSKA H, SUZUKI T S, et al. High- temperature flexural strength performance of ternary high-entropy carbide consolidated via spark plasma sintering of TaC, ZrC and NbC. Scripta Mater., 2019,164:12-16. |
| [16] | LI F, BAO W C, SUN S K, et al. Synthesis of single-phase metal oxycarbonitride ceramics. Scripta Mater., 2020,176:17-22. |
| [17] | KUMAR A, GUPTA M. An insight into evolution of light weight high entropy alloys: a review. Metals Basel, 2016,6(9):199. |
| [18] | ROST C M, SACHET E, BORMAN T, et al. Entropy-stabilized oxides. Nat. Commun., 2015,6(1):8485. |
| [19] | WEI X F, QIN Y, LIU J X, et al. Gradient microstructure development and grain growth inhibition in high-entropy carbide ceramics prepared by reactive spark plasma sintering . J. Eur. Ceram. Soc., 2020,40(4):935-941. |
| [20] | LI F, LU Y, WANG X G, et al. Liquid precursor-derived high- entropy carbide nanopowders. Ceram. Int., 2019,45(7):22437-22441. |
| [21] | WEI X F, LIU J X, LI F, et al. High entropy carbide ceramics from different starting materials. J. Eur. Ceram. Soc., 2019,39(10):2989-2994. |
| [22] | DUSZA J, SVEC P, GIRMAN V, et al. Microstructure of (Hf-Ta-Zr-Nb)C high-entropy carbide at micro and nano/atomic level. J. Eur. Ceram. Soc., 2018,38(12):4303-4307. |
| [23] | ZHOU J Y, ZHANG J Y, ZHANG F, et al. High-entropy carbide: a novel class of multicomponent ceramics. Ceram. Int., 2018,44(17):22014-22018. |
| [24] | JIANG S C, HU T, GILD J, et al. A new class of high-entropy perovskite oxides. Scripta Mater., 2018,142:116-120. |
| [25] | ZHAO Z F, CHEN H, XIANG H M, et al. (Y0.25Yb0.25Er0.25Lu0.25)2(Zr0.5Hf0.5)2O7: a defective fluorite structured high entropy ceramic with low thermal conductivity and close thermal expansion coefficient to Al2O3. J. Mater. Sci. Technol., 2020,39:167-172. |
| [26] | DABROWA J, STYGAR M, MIKULA A, et al. Synthesis and microstructure of the (Co,Cr,Fe,Mn,Ni)3O4 high entropy oxide characterized by spinel structure. Mater. Lett., 2018,216:32-36. |
| [27] | ZHAO Z F, CHEN H, XIANG H M, et al. (La0.2Ce0.2Nd0.2Sm0.2Eu0.2)PO4: a high-entropy rare-earth phosphate monazite ceramic with low thermal conductivity and good compatibility with Al2O3. J. Mater. Sci. Technol., 2020,38:80-85. |
| [28] | GILD J, KAUFMANN K, Vecchio K, et al. Reactive flash spark plasma sintering of high-entropy ultrahigh temperature ceramics. Scripta. Mater., 2019,170:106-110. |
| [29] | YAN J L, LIU F S, MA G H, et al. Suppression of the lattice thermal conductivity in NbFeSb-based half-Heusler thermoelectric materials through high entropy effects. Scripta Mater., 2018,157:129-134. |
| [30] | GILD J, BRAUN J L, KAUFMANN K, et al. A high-entropy silicide: (Mo0.2Nb0.2Ta0.2Ti0.2W0.2)Si2. J. Mater., 2019,5(3):337-343. |
| [31] |
SARKER P, HARRINGTON T, TOHER C, et al. High-entropy high-hardness metal carbides discovered by entropy descriptors. Nat. Commun., 2018,9(1):4980.
URL PMID |
| [32] |
SARKAR A, VELASCO L, WANG D, et al. High entropy oxides for reversible energy storage. Nat. Commun., 2018,9(1):3400.
URL PMID |
| [33] |
WANG H X, CAO Y J, LIU W, et al. Oxidation behavior of (Hf0.2Ta0.2Zr0.2Ti0.2Nb0.2)C-xSiC ceramics at high temperature. Ceram. Int., 2020,46(8):11160-11168.
DOI URL |
| [34] | TAN Y Q, CHEN C, Li S G, et al. Oxidation behaviours of high-entropy transition metal carbides in 1200 ℃ water vapor. J. Alloys Compd., 2020,816:152523. |
| [35] | REN K, WANG Q K, SHAO G, et al. Multicomponent high- entropy zirconates with comprehensive properties for advanced thermal barrier coating. Scripta Mater., 2020,178:382-386. |
| [36] | DONG Y, REN K, LU Y H, et al. High-entropy environmental barrier coating for the ceramic matrix composites. J. Eur. Ceram. Soc., 2018,39(7):2574-2579. |
| [37] | TENG Z, ZHU L N, TAN Y Q, et al. Synjournal and structures of high-entropy pyrochlore oxides. J. Eur. Ceram. Soc., 2020,40(4):1639-1643. |
| [38] | ZHAO Z F, XIANG H M, DAI F Z, et al. (La0.2Ce0.2Nd0.2Sm0.2Eu0.2)2Zr2O7: A novel high-entropy ceramic with low thermal conductivity and sluggish grain growth rate. J. Mater. Sci. Technol., 2019,35(11):2647-2651. |
| [39] | SAVINO R, FUMO M D S, PATERNA D, et al. Aerothermodynamic study of UHTC-based thermal protection systems. Aerosp. Sci. Technol., 2005,9(2):151-160. |
| [40] | OPRKA M M, TALMY I G, ZAYKOSKI J A. Oxidation-based materials selection for 2000 ℃ + hypersonic aerosurfaces: theoretical considerations and historical experience. J. Mater. Sci., 2004,39(19):5887-5904. |
| [41] | KUBOTA Y, YANO M, INOUE R, et al. Oxidation behavior of ZrB2-SiC-ZrC in oxygen-hydrogen torch environment. J. Eur. Ceram. Soc., 2017,38(4):1095-1102. |
| [42] | RAMA RAO G A, VENUGOPAL V. Kinetics and mechanism of the oxidation of ZrC. J. Alloys Compd., 1994,206(2):237-242. |
| [43] | VOITOVICH R F, PUGACH E A. High-temperature oxidation of ZrC and HfC. Powder Metall. Met. C, 1973,12(11):916-921. |
| [44] | CHEN L Y, GU Y L, SHI L, et al. Synjournal and oxidation of nanocrystalline HfB2. J. Alloys Compd., 2004,368(1):353-356. |
| [45] | SHIMADA S. Interfacial reaction on oxidation of carbides with formation of carbon. Solid State Ionics, 2001,141:99-104. |
| [46] | PARTHASARATHY T A, RAPP R A, OPEKA M M, et al. A model for the oxidation of ZrB2, HfB2 and TiB2. Acta Mater., 2007,55(17):5999-6010. |
| [47] | PARTHASARATHY T A, RAPP R A, OPEKA M M, et al. Effect of phase change and oxygen permeability in oxide scales on oxidation kinetics of ZrB2 and HfB2. J. Am. Ceram. Soc., 2009,92(5):1079-1086. |
| [48] | JING Y, YUAN H B, LIAN Z S. Microstructure and mechanical properties of ZrB2-HfC ceramics influenced by HfC addition. Materials, 2018,11(10):2046. |
| [49] | MALLIK M, RAY K K, MITRA R. Oxidation behavior of hot pressed ZrB2-SiC and HfB2-SiC composites. J. Eur. Ceram. Soc., 2011,31(1):199-215. |
| [50] | TRIPP W C, GRAHAM H C. Thermogravimetric study of oxidation of ZrB2 in temperature range of 800 ℃ to 1500 ℃. J. Electrochem. Soc., 1971,118(7):1195-1199. |
| [51] | FAHRENHOLTZ W G. The ZrB2 volatility diagram. J. Am. Ceram. Soc., 2005,88(12):3509-3512. |
| [52] | FAHRENHOLTZ W G. Thermodynamic analysis of ZrB2-SiC oxidation: formation of a SiC-depleted region . J. Am. Ceram. Soc., 2007,90(1):143-148. |
| [53] | HU P, GUOLIN W, WANG Z. Oxidation mechanism and resistance of ZrB2-SiC composites. Corros. Sci., 2009,51(11):2724-2732. |
| [54] | JACOBSON N S, MYERS D L. Active oxidation of SiC. Oxid. Met., 2011,75(1):1-25. |
| [55] | JACOBSON N S, HARDER B, MYERS D L, et al. Oxidation transitions for SiC. Part I. Active-to-passive transitions. J. Am. Ceram. Soc., 2013,96(3):838-844. |
| [56] | WANG Y G, LUO L, SUN J, et al. ZrB2-SiC(Al) ceramics with high resistance to oxidation at 1500 ℃. Corros. Sci., 2013,74:154-158. |
| [57] | HE J B, WANG Y G, LUO L, et al. Oxidation behaviour of ZrB2-SiC (Al/Y) ceramics at 1700 ℃. J. Eur. Ceram. Soc., 2016,36(15):3769-3774. |
| [58] | WANG Y G, MA B S, LI L L, et al. Oxidation behavior of ZrB2-SiC-TaC ceramics . J. Am. Ceram. Soc., 2012,95(1):374-378. |
| [59] |
TONG Z W, HE R J, CHENG T B, et al. High temperature oxidation behavior of ZrB2-SiC added MoSi2 ceramics. Ceram. Int., 2018,44(17):21076-21082.
DOI URL |
| [60] | ZAPATASOLVAS E, JAYASEELAN D D, BROWN P, et al. Effect of La2O3 addition on long-term oxidation kinetics of ZrB2-SiC and HfB2-SiC ultra-high temperature ceramics . J. Eur. Ceram. Soc., 2014,34(15):3535-3548. |
| [61] | GILD J, ZHANG Y Y, HARRINGTON T, et al. High-entropy metal diborides: a new class of high-entropy materials and a new type of ultrahigh temperature ceramics. Sci. Rep-UK, 2016,6(1):37946-37946. |
| [62] | YE B L, WEN T Q, HUANG K H, et al. First-principles study, fabrication, and characterization of (Hf0.2Zr0.2Ta0.2Nb0.2Ti0.2)C high- entropy ceramic. J. Am. Ceram. Soc., 2019,102(7):4344-4352. |
| [63] | HOSKING F M. Sodium compatibility of refractory-metal alloy- type 304l stainless-steel joints. Int. J. Refract. Met. H., 1985,64(7):S181-S190. |
| [64] | WERNER E A. Introduction to the thermodynamics of materials. Mat. Sci. Eng., 2008,494(1/2):464. |
| [65] |
CAR R, PARRINELLO M. Unified approach for molecular dynamics and density-functional theory. Phys. Rev. Lett., 1985,55(22):2471-2474.
URL PMID |
| [66] | LIU X J, WANG C P, GAO F, et al. Thermodynamic calculation of phase equilibria in the Sn-Ag-Cu-Ni-Au System . J. Electron. Mater., 2007,36(11):1429-1441. |
| [67] | WANG C P, WANG J, GUO S H, et al. Experimental investigation and thermodynamic calculation of the phase equilibria in the Co-Mo-W system. Intermetallics, 2009,17(8):642-650. |
| [68] | FENG R, GAO M C, LEE C, et al. Design of light-weight high- entropy alloys. Entropy Switz., 2016,18(9):333-353. |
| [69] | KIM J. Applicability of special quasi-random structure models in thermodynamic calculations using semi-empirical Debye-Grüneisen theory . J. Alloys Compd., 2015,650:564-571. |
| [70] | VOAS B K, USHER T M, LIU X M, et al. Special quasirandom structures to study the (K0.5Na0.5)NbO3 random alloy. Phys. Rev. B, 2014,90(2):024105-1-6. |
| [71] | SAHARA R, EMURA S, LI S, et al. First-principles study of electronic structures and stability of body-centered cubic Ti-Mo alloys by special quasirandom structures. Sci. Technol. Adv. Mat., 2014,15(3):035014-1-10. |
| [72] |
VITOS L, ABRIKOSOV I A, JOHANSSON B. Anisotropic lattice distortions in random alloys from first-principles theory. Phys. Rev. Lett., 2001,87(15):156401-1-4.
URL PMID |
| [73] | ABRIKOSOV I A, JOHANSSON B. Applicability of the coherent- potential approximation in the theory of random alloys. Phys. Rev. B, 1998,57(22):14164-14173. |
| [74] | YE B L, WEN T Q, NGUYEN M C, et al. First-principles study, fabrication and characterization of (Zr0.25Nb0.25Ti0.25V0.25)C high- entropy ceramics. Acta Mater., 2019,170:15-23. |
| [75] |
BRAIC V, VLADESCU A, BALACEANU M, et al. Nanostructured multi-element (TiZrNbHfTa)N and (TiZrNbHfTa)C hard coatings. Surf. Coat. Tech., 2012,211:117-121.
DOI URL |
| [76] |
LIN S Y, CHANG S Y, HUANG Y C, et al. Mechanical performance and nanoindenting deformation of (AlCrTaTiZr)NCy multi-component coatings co-sputtered with bias. Surf. Coat. Tech., 2012,206(24):5096-5102.
DOI URL |
| [77] |
ZHOU J Y, ZHANG J Y, ZHANG F, et al. high-entropy carbide: a novel class of multicomponent ceramics. Ceram. Int., 2018,44(17):22014-22018.
DOI URL |
| [78] |
FENG L, FAHRENHOLTZ W G, HILMAS G E, et al. Synthesis of single-phase high-entropy carbide powders. Scripta Mater., 2019,162(12):90-93.
DOI URL |
| [79] | YE B L, NING S S, LIU D, et al. One-step synthesis of coral-like high-entropy metal carbide powders. J.Am. Ceram. Soc., 102(10):6372-6378. |
| [80] |
DU B, LIU H H, CHU Y H. Fabrication and characterization of polymer-derived high-entropy carbide ceramic powders . J. Am. Ceram. Soc., 2020,103:4063-4068.
DOI URL |
| [81] | NING S S, WEN T Q, YE B L, et al. Low-temperature molten salt synjournal of high-entropy carbide nanopowders . J. Am. Ceram. Soc., 2020,103(3):2244-2251. |
| [82] | JAGADEESH S, VISHNU D S M, KIM H K, et al. Facile electrochemical synthesis of nanoscale (TiNbTaZrHf)C high-entropy carbide powder. Angew. Chem. Int. Ed., 2020 59(29):11830-11835. |
| [83] | BRAIC M, BRAIC V, BALACEANU M, et al. Characteristics of (TiAlCrNbY)C films deposited by reactive magnetron sputtering. Surf. Coat. Tech., 2010,204(12):2010-2014. |
| [84] | JHONG Y S, HUANG C W, LIN S J, et al. Effects of CH4 flow ratio on the structure and properties of reactively sputtered (CrNbSiTiZr)Cx coatings. Mater. Chem. Phys., 2017,210:348-352. |
| [85] | BRAIC M, BALACEANU M, VLADESCU A, et al. Deposition and characterization of multi-principal-element (CuSiTiYZr)C coatings. Appl. Surf. Sci., 2013 284:671-678. |
| [86] | BRAIC V, PARAU A C, PANA I, et al. Effects of substrate temperature and carbon content on the structure and properties of (CrCuNbTiY)C multicomponent coatings. Surf. Coat. Tech., 2014 258:996-1005. |
| [87] | CSANADI T, CASTLE E G, REECE M J, et al. Strength enhancement and slip behaviour of high-entropy carbide grains during micro-compression. Sci. Rep-UK, 2019,9(1):10200. |
| [88] | WANG C, YE Y, GUAN X, et al. An analysis of tribological performance on Cr/GLC film coupling with Si3N4, SiC, WC, Al2O3 and ZrO2 in seawater. Tribol. Int., 2016 96:77-86. |
| [89] | HARRINGTON T J, GILD J, SARKER P, et al. Phase stability and mechanical properties of novel high entropy transition metal carbides. Acta Mater., 2019 166:271-280. |
| [90] | WANG K, CHEN L, XU C G, et al. Microstructure and mechanical properties of (TiZrNbTaMo)C high-entropy ceramic. J. Mater. Sci. Technol., 2020,39:99-105. |
| [91] | HAN X X, VLADIMIR G, RICHARD S, et al. Improved creep resistance of high entropy transition metal carbides . J. Eur. Ceram. Soc., 2020,40(7):2709-2715. |
| [92] | YAN X L, CONSTANTIN L, LU Y F, et al. (Hf0.2Zr0.2Ta0.2Nb0.2Ti0.2)C high-entropy ceramics with low thermal conductivity. J. Am. Ceram. Soc., 2018,101(10):4486-4491. |
| [93] | CHEN H, XIANG H M, DAI F Z, et al. Porous high entropy (Zr0.2Hf0.2Ti0.2Nb0.2Ta0.2)B2: a novel strategy towards making ultrahigh temperature ceramics thermal insulating. J. Mater. Sci. Technol., 2019,35(10):2404-2408. |
| [94] | CHEN H, XIANG H M, DAI F Z, et al. High porosity and low thermal conductivity high entropy (Zr0.2Hf0.2Ti0.2Nb0.2Ta0.2)C. J. Mater. Sci. Technol., 2019,35(8):1700-1705. |
| [95] |
YE B L, WEN T Q, LIU D, et al. Oxidation behavior of (Hf0.2Zr0.2Ta0.2Nb0.2Ti0.2)C high-entropy ceramics at 1073-1473 K in air. Corros. Sci., 2019,153:327-332.
DOI URL |
| [96] | YE B L, WEN T Q, CHU Y H. High-temperature oxidation behavior of (Hf0.2Zr0.2Ta0.2Nb0.2Ti0.2)C high-entropy ceramics in air. J. Am. Ceram. Soc., 2019,103(1):500-507. |
| [97] | WANG H X, HAN X, LIU W, et al. Oxidation behavior of high-entropy carbide (Hf0.2Ta0.2Er0.2Ti0.2Nb0.2)C at 1400-1600 ℃. DOI: 10.1016/j.ceramint.2020.12.201. |
| [98] | BACKMAN L, GILD J, LUO J, et al. Theoretical predictions of preferential oxidation in refractory high entropy materials. Acta Mater., 2020,197:20-27. |
| [99] | WANG H X, WANG S Y, CAO Y J, et al. Oxidation behaviors of (Hf0.25Zr0.25Ta0.25Nb0.25)C and (Hf0.25Zr0.25Ta0.25Nb0.25)C-SiC at 1300-1500 ℃. J. Mater. Sci. Technol., 2021,60:147-155. |
| [100] |
BRAIC V, BALACEANU M, BRAIC M, et al. Characterization of multi-principal-element (TiZrNbHfTa)N and (TiZrNbHfTa)C coatings for biomedical applications. J. Mech. Behav. Biomed., 2019,10:197-205.
DOI URL |
| [101] | WANG F, YAN X L, WANG T Y, et al. Irradiation damage in (Zr0.25Ta0.25Nb0.25Ti0.25)C high-entropy carbide ceramics. Acta Mater., 2020,195:739-749. |
| [1] | DING Ling, JIANG Rui, TANG Zilong, YANG Yunqiong. MXene: Nanoengineering and Application as Electrode Materials for Supercapacitors [J]. Journal of Inorganic Materials, 2023, 38(6): 619-633. |
| [2] | YANG Zhuo, LU Yong, ZHAO Qing, CHEN Jun. X-ray Diffraction Rietveld Refinement and Its Application in Cathode Materials for Lithium-ion Batteries [J]. Journal of Inorganic Materials, 2023, 38(6): 589-605. |
| [3] | CHEN Qiang, BAI Shuxin, YE Yicong. Highly Thermal Conductive Silicon Carbide Ceramics Matrix Composites for Thermal Management: a Review [J]. Journal of Inorganic Materials, 2023, 38(6): 634-646. |
| [4] | LIN Junliang, WANG Zhanjie. Research Progress on Ferroelectric Superlattices [J]. Journal of Inorganic Materials, 2023, 38(6): 606-618. |
| [5] | ZHANG Shuo, FU Qiangang, ZHANG Pei, FEI Jie, LI Wei. Influence of High Temperature Treatment of C/C Porous Preform on Friction and Wear Behavior of C/C-SiC Composites [J]. Journal of Inorganic Materials, 2023, 38(5): 561-568. |
| [6] | NIU Jiaxue, SUN Si, LIU Pengfei, ZHANG Xiaodong, MU Xiaoyu. Copper-based Nanozymes: Properties and Applications in Biomedicine [J]. Journal of Inorganic Materials, 2023, 38(5): 489-502. |
| [7] | YUAN Jingkun, XIONG Shufeng, CHEN Zhangwei. Research Trends and Challenges of Additive Manufacturing of Polymer-derived Ceramics [J]. Journal of Inorganic Materials, 2023, 38(5): 477-488. |
| [8] | DU Jianyu, GE Chen. Recent Progress in Optoelectronic Artificial Synapse Devices [J]. Journal of Inorganic Materials, 2023, 38(4): 378-386. |
| [9] | YANG Yang, CUI Hangyuan, ZHU Ying, WAN Changjin, WAN Qing. Research Progress of Flexible Neuromorphic Transistors [J]. Journal of Inorganic Materials, 2023, 38(4): 367-377. |
| [10] | YOU Junqi, LI Ce, YANG Dongliang, SUN Linfeng. Double Dielectric Layer Metal-oxide Memristor: Design and Applications [J]. Journal of Inorganic Materials, 2023, 38(4): 387-398. |
| [11] | QI Zhanguo, LIU Lei, WANG Shouzhi, WANG Guogong, YU Jiaoxian, WANG Zhongxin, DUAN Xiulan, XU Xiangang, ZHANG Lei. Progress in GaN Single Crystals: HVPE Growth and Doping [J]. Journal of Inorganic Materials, 2023, 38(3): 243-255. |
| [12] | ZHANG Chaoyi, TANG Huili, LI Xianke, WANG Qingguo, LUO Ping, WU Feng, ZHANG Chenbo, XUE Yanyan, XU Jun, HAN Jianfeng, LU Zhanwen. Research Progress of ScAlMgO4 Crystal: a Novel GaN and ZnO Substrate [J]. Journal of Inorganic Materials, 2023, 38(3): 228-242. |
| [13] | CHEN Kunfeng, HU Qianyu, LIU Feng, XUE Dongfeng. Multi-scale Crystallization Materials: Advances in in-situ Characterization Techniques and Computational Simulations [J]. Journal of Inorganic Materials, 2023, 38(3): 256-269. |
| [14] | LIN Siqi, LI Airan, FU Chenguang, LI Rongbing, JIN Min. Crystal Growth and Thermoelectric Properties of Zintl Phase Mg3X2 (X=Sb, Bi) Based Materials: a Review [J]. Journal of Inorganic Materials, 2023, 38(3): 270-279. |
| [15] | LIU Yan, ZHANG Keying, LI Tianyu, ZHOU Bo, LIU Xuejian, HUANG Zhengren. Electric-field Assisted Joining Technology for the Ceramics Materials: Current Status and Development Trend [J]. Journal of Inorganic Materials, 2023, 38(2): 113-124. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||