
Journal of Inorganic Materials ›› 2020, Vol. 35 ›› Issue (8): 895-901.DOI: 10.15541/jim20190606
Special Issue: 功能材料论文精选(一):光学材料(2020)
• RESEARCH PAPER • Previous Articles Next Articles
LI Shufang( ),ZHAO Shuang,ZHOU Xiao,LI Manrong(
),ZHAO Shuang,ZHOU Xiao,LI Manrong( )
)
Received:2019-11-29
Revised:2020-01-16
Published:2020-08-20
Online:2020-03-06
Supported by:CLC Number:
LI Shufang,ZHAO Shuang,ZHOU Xiao,LI Manrong. Crystal Structures, Optical, and Magnetic Properties of Zn3-xMnxTeO6[J]. Journal of Inorganic Materials, 2020, 35(8): 895-901.
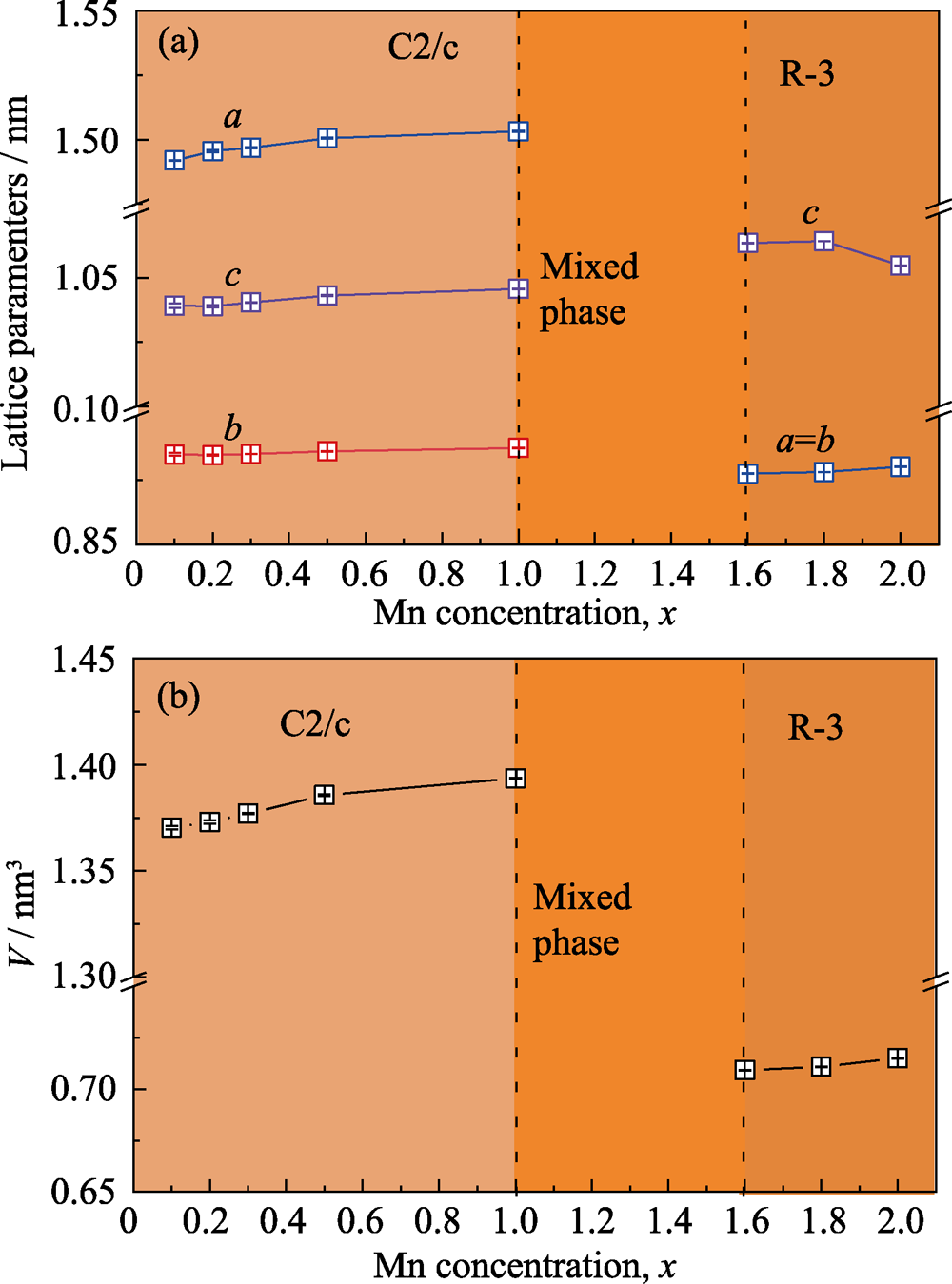
Fig. 2 Lattice parameters a, b and c [nm] (a), and V [nm3] (b) obtained from Zn3-xMnxTeO6 (0<x≤2.0) solid solutions by XRD measurements at room temperature
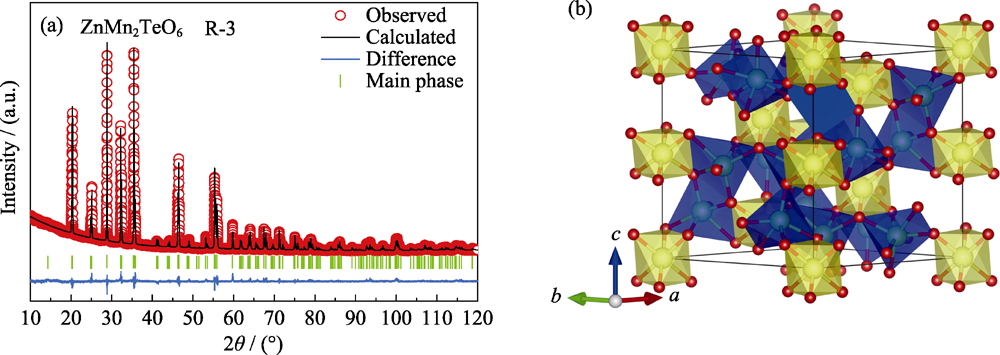
Fig. 3 Rietveld refined of the PXRD data (a) and crystal structure (b) of ZnMn2TeO6 Zn/Mn: blue spheres; Te: yellow spheres; O: red spheres; TeO6 octahedra: yellow; MnO6 octahedra: blue
| Atom | Site | x | y | z | B/nm2 | Occ. |
|---|---|---|---|---|---|---|
| Mn/Zn | 18f | 0.0388(1) | 0.2240(1) | 0.2864(1) | 0.00510(1) | 0.667/0.333 |
| Te1 | 3b | 0 | 0 | 0.5 | 0.00710(1) | 1.0 |
| Te2 | 3a | 0 | 0 | 0 | 0.00320(1) | 1.0 |
| O1 | 18f | 0.1773(4) | 0.1972(4) | 0.3833(1) | 0.00510(1) | 1.0 |
| O2 | 18f | 0.2061(3) | 0.0348(5) | 0.1071(2) | 0.00550(1) | 1.0 |
Table 1 Crystallographic parameters for ZnMn2TeO6 obtained from the Rietveld refinement of the PXRD data
| Atom | Site | x | y | z | B/nm2 | Occ. |
|---|---|---|---|---|---|---|
| Mn/Zn | 18f | 0.0388(1) | 0.2240(1) | 0.2864(1) | 0.00510(1) | 0.667/0.333 |
| Te1 | 3b | 0 | 0 | 0.5 | 0.00710(1) | 1.0 |
| Te2 | 3a | 0 | 0 | 0 | 0.00320(1) | 1.0 |
| O1 | 18f | 0.1773(4) | 0.1972(4) | 0.3833(1) | 0.00510(1) | 1.0 |
| O2 | 18f | 0.2061(3) | 0.0348(5) | 0.1071(2) | 0.00550(1) | 1.0 |
| [1] |
TOKURA Y, SEKI S, NAGAOSA N. Multiferroics of spin origin. Reports Progress in Physics, 2014,77:076501.
DOI URL |
| [2] |
EERENSTEIN W, MATHUR N D, SCOTT J F. Multiferroic and magnetoelectric materials. Nature, 2006,442:759-765.
DOI URL PMID |
| [3] |
RAO C N R, SUNDARESAN A, SAHA R. Multiferroic and magnetoelectric oxides: the emerging scenario. The Journal of Physical Chemistry Letters, 2012,3(16):2237-2246.
DOI URL PMID |
| [4] |
SPALDIN N A, FIEBIG M. The renaissance of magnetoelectric multiferroics. Science, 2005,309(5733):391-392.
URL PMID |
| [5] |
KIMURA T, GOTO T, SHINTANI H, et al. Magnetic control of ferroelectric polarization. Nature, 2003,426(6962):55-58.
DOI URL PMID |
| [6] |
WANG J, NEATON J B, ZHENG H, et al. Epitaxial BiFeO3 multiferroic thin film heterostructures. Science, 2003,299:1719-1722.
DOI URL PMID |
| [7] |
KITAGAWA Y, HIRAOKA Y, HONDA T, et al. Low-field magnetoelectric effect at room temperature. Nature Materials, 2010,9(10):797-802.
DOI URL PMID |
| [8] |
LI M R, ADEM U, MCMITCHELL S R C, et al. A polar corundum oxide displaying weak ferromagnetism at room temperature. Journal of the American Chemical Society, 2012,134(8):3737-3747.
DOI URL PMID |
| [9] | FINGER L W, HAZEN R M. Crystal structure and compression of ruby to 46 kbar. Journal of Applied Physics (USA), 1978,49(12):5823-5826. |
| [10] | CAI G H, GREEBLATT M, LI M R. Polar magnets in double corundum oxides. Chemistry of Materials, 2017,29(13):5447-5457. |
| [11] | SCHULZ H, BAYER G. Structure determination of Mg3TeO6. Acta Crystallographica,Section B (Structural Crystallography and Crystal Chemistry), 1971,B27:815-21. |
| [12] | WEIL M. Mn3TeO6. Acta Crystallographica Section E Structure Reports Online, 2006,62(12):i244-i245. |
| [13] | BECKER R, BERGER H. Reinvestigation of Ni3TeO6. Acta Crystallographica Section E-Structure Reports Online, 2006,62:I222-I223. |
| [14] | FALCK L, LINDQVIST O, MORET J. Tricopper(II) tellurate(VI). Acta Crystallographica Section B, 1978,34(3):896-897. |
| [15] | HERAK M. Cubic magnetic anisotropy of the antiferromagnetically ordered Cu3TeO6. Solid State Communications, 2011,151(21):1588-1592. |
| [16] | MATHIEU R, IVANOV S A, NORDBLAD P, et al. Enhancement of antiferromagnetic interaction and transition temperature in M3TeO6 systems (M=Mn, Co, Ni, Cu). The European Physical Journal B, 2013,86(8):361. |
| [17] | CAIMI G, DEGIORGI L, BERGER H, et al. Optical evidence for a magnetically driven structural transition in the spin web Cu3TeO6. Europhysics Letters, 2006,75(3):496. |
| [18] | BHIM A, GOPALAKRISHNAN J, NATARAJAN S. Exploring the corundum structure as a host for colored compounds-synthesis, structures, and optical studies of (MM’)3TeO6(M=Mg, Mn, Co, Ni, Zn; M’=Mg, Mn, Co, Ni, Cu). European Journal of Inorganic Chemistry, 2018(20/21):2277-2284. |
| [19] | WEIL M. Zn3TeO6. Acta Crystallographica Section E Structure Reports Online, 2006,62(12):i246-i247. |
| [20] | IVANOV S A, TELLGREN R, RITTER C, et al. Temperature- dependent multi-k magnetic structure in multiferroic Co3TeO6. Materials Research Bulletin, 2012,47(1):63-72. |
| [21] | IVANOV S A, MATHIEU R, NORDBLAD P, et al. Spin and dipole ordering in Ni2InSbO6 and Ni2ScSbO6 with corundum-related structure. Chemistry of Materials, 2013,22(6):935-945. |
| [22] | CHOI K Y, LEMMENS P, CHOI E S, et al. Lattice anomalies and magnetic excitations of the spin web compound Cu3TeO6. Journal of Physics: Condensed Matter, 2008,20(50):505214. |
| [23] | SINGH H, GHOSH H, CHANDRASEKHAR RAO T V, et al. Short range ferromagnetic, magneto-electric, and magneto- dielectric effect in ceramic Co3TeO6. Journal of Applied Physics, 2016,119(4):044104. |
| [24] | SINGH H, SINHA A K, GHOSH H, et al. Structural investigations on Co3-xMnxTeO6; (0<x≤2): high temperature ferromagnetism and enhanced low temperature anti-ferromagnetism. Journal of Applied Physics (USA), 2014, 116 (7): 074904-1-9. |
| [25] | HARRIS A B. Symmetry analysis of multiferroic Co3TeO6. Physical Review B, 2012,85(10):100403. |
| [26] | HER J L, CHOU C C, MATSUDA Y H, et al. Magnetic phase diagram of the antiferromagnetic cobalt tellurate Co3TeO6. Physical Review B, 2011,84(23):35123. |
| [27] | TOLEDANO P, CAROLUS V, HUDL M, et al. First-order multi-k phase transitions and magnetoelectric effects in multiferroic Co3TeO6. Physical Review B, 2012,85(21):214439. |
| [28] | WANG C W, LEE C H, LI C Y, et al. Complex magnetic couplings in Co3TeO6. Physical Review B, 2013,88(18):184427. |
| [29] | HUDL M, MATHIEU R, IVANOV S A, et al. Complex magnetism and magnetic-field-driven electrical polarization of Co3TeO6. Physical Review B, 2011,84(18):180404. |
| [30] | HARRIS A B. Symmetry analysis of multiferroic Co3TeO6. Physical Review B, 2012,85(10):100403. |
| [31] | SINGH H, GHOSH H, RAO T V C, et al. Observation of high-spin mixed oxidation state of cobalt in ceramic Co3TeO6. Journal of Applied Physics, 2014, 116(21): 214106-1-7. |
| [32] |
IVANOV S A, NORDBLAD P, MATHIEU R, et al. New type of incommensurate magnetic ordering in Mn3TeO6. Materials Research Bulletin, 2011,46(11):1870-1877.
DOI URL |
| [33] |
IVANOV S A, MATHIEU R, NORDBLAD P, et al. Chemical pressure effects on structural, dielectric and magnetic properties of solid solutions Mn3-xCoxTeO6. Materials Research Bulletin, 2014,50:42-56.
DOI URL |
| [34] |
COELHO A. Whole-profile structure solution from powder diffraction data using simulated annealing. Journal of Applied Crystallography, 2000,33(3):899-908.
DOI URL |
| [35] |
TAUC J. Optical properties and electronic structure of amorphous Ge and Si. Materials Research Bulletin, 1968,3(1):37-46.
DOI URL |
| [36] |
LIU C, YE M, HAN A, et al. Structural analysis and characterization of doped spinel Co2-xMxTiO4 (M=Mg 2+, Mn 2+, Ni 2+, Cu 2+ and Zn 2+) coated mica composite pigments . Ceramics International, 2015,41(4):5537-5546.
DOI URL |
| [1] | ZHAO Wei, XU Yang, WAN Yingjie, CAI Tianxun, MU Jinxiao, HUANG Fuqiang. Metal Cyanamides/Carbodiimides: Structure, Synthesis and Electrochemical Energy Storage Performance [J]. Journal of Inorganic Materials, 2022, 37(2): 140-151. |
| [2] | HU Jingsan, GU Jianfei, ZHANG Weiyi. Mechanism of the Magnetic and Specific-heat Anomalies in Rare-earth Dodecaborides RB12 (R=Tb-Tm): an Effect of Crystal-field-splitting Order Parameter [J]. Journal of Inorganic Materials, 2021, 36(8): 865-870. |
| [3] | PENG Fan, ZENG Yi. Method of Crystal Structure Identification by Using Kikuchi Diffraction Patterns [J]. Journal of Inorganic Materials, 2021, 36(11): 1193-1198. |
| [4] | LI Shufang, ZHAO Shuang, LI Manrong. Flux Growth of Tungsten Oxychloride Li23CuW10O40Cl5 [J]. Journal of Inorganic Materials, 2020, 35(7): 834-838. |
| [5] | HUANG Chong,ZHAO Wei,WANG Dong,BU Kejun,WANG Sishun,HUANG Fuqiang. Synthesis, Crystal Structure, and Electrical Conductivity of Pd-intercalated NbSe2 [J]. Journal of Inorganic Materials, 2020, 35(4): 505-510. |
| [6] | Xiang-Xiong ZENG, Jin-Chao YANG, Lian ZUO, Ben-Ben YANG, Jun QIN, Zhi-Hang PENG. Li/Ce/La Multidoping on Crystal Structure and Electric Properties of CaBi2Nb2O9 Piezoceramics [J]. Journal of Inorganic Materials, 2019, 34(4): 379-386. |
| [7] | HUANG Long, DING Shi-Hua, ZHANG Xiao-Yun, YAN Xin-Kan, LI Chao, ZHU Hui. Structure and Microwave Dielectric Property of BaAl2Si2O8 with Li2O-B2O3-SiO2 Glass Addition [J]. Journal of Inorganic Materials, 2019, 34(10): 1091-1096. |
| [8] | CHEN Wei-Bin, LIU Xue-Chao, ZHUO Shi-Yi, CHAI Jun, SHI Er-Wei. Influence of Proton Irradiation on Defect and Magnetism of Yb-doped ZnO Dluted Magnetic Semiconductor Thin Films [J]. Journal of Inorganic Materials, 2018, 33(8): 903-908. |
| [9] | ZHOU Xin, MA Lei, LIU Tao, GUO Yong-Bin, WANG Dao, DONG Pei-Lin. Crystal Structure and Magnetic Property of Si3N4/FePd/Si3N4 Thin Films [J]. Journal of Inorganic Materials, 2018, 33(8): 909-913. |
| [10] | MENG Fan-Bin, MA Xiao-Fan, ZHANG Wei, WU Guang-Heng, ZHANG Yu-Jie. Structure and Magnetic Property of Fe and Mn Doped Spinel Co2MnO4 [J]. Journal of Inorganic Materials, 2017, 32(6): 609-614. |
| [11] | ZHANG Lin, LIU Hui-Hui, LIU Lin-Jia, ZHAO Guo-Qing, WU Yan, MIN Guang-Hui. Effects of La Doping on CaB6 Thin Films Prepared by DC Magnetron Sputtering [J]. Journal of Inorganic Materials, 2017, 32(5): 555-560. |
| [12] | WANG Qing-Qing, SHI Jian, LI Huan-Ying, CHEN Xiao-Feng, PAN Shang-Ke, BIAN Jian-Jiang, REN Guo-Hao. Optical and Scintillation Properties of Cs2LiYCl6:Ce Crystal [J]. Journal of Inorganic Materials, 2017, 32(2): 175-179. |
| [13] | DAN Meng, ZHANG Qian, ZHONG Yun-Qian, ZHOU Ying. Preparation of MnS with Different Crystal Phases for Photocatalytic H2 Production from H2S [J]. Journal of Inorganic Materials, 2017, 32(12): 1308-1314. |
| [14] | YANG Zhi-Sheng, KE Wei-Fang, WANG Yan-Xiang, HUANG Li-Qun, GUO Ping-Chun, ZHU Hua. Preparation and Characterization of a Novel Hybrid Perovskite (HOC2H4NH3)2CuCl4 [J]. Journal of Inorganic Materials, 2017, 32(10): 1063-1067. |
| [15] | ZHANG Yao, DING Shi-Hua, LIU Yang-Qiong, DUAN Shao-Ying, XIAO Peng, HAN Lin-Cai. Crystal Structure and Microwave Dielectric Property of Ba1-xMgxAl2Si2O8 [J]. Journal of Inorganic Materials, 2017, 32(1): 91-95. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||