
Journal of Inorganic Materials ›› 2020, Vol. 35 ›› Issue (9): 1005-1010.DOI: 10.15541/jim20190444
Special Issue: 环境材料论文精选(2020); 【虚拟专辑】污染物吸附水处理(2020~2021)
• RESEARCH PAPER • Previous Articles Next Articles
LI Jing1,2( ),LIU Xiaoyue2,QIU Qianfeng2,LI Ling2,CAO Xiaoyan1,2,3(
),LIU Xiaoyue2,QIU Qianfeng2,LI Ling2,CAO Xiaoyan1,2,3( )
)
Received:2019-08-28
Revised:2020-01-13
Published:2020-09-20
Online:2020-03-03
Supported by:CLC Number:
LI Jing,LIU Xiaoyue,QIU Qianfeng,LI Ling,CAO Xiaoyan. Phosphorus Sorption Characteristics on Aluminum Oxides with Different Structures[J]. Journal of Inorganic Materials, 2020, 35(9): 1005-1010.
| NaNO3 concentration/ (mol·L-1) | HS concentration/(mol·L-1) | TOTHpH=8/(mmol·L-1) | pHPZNPC* | |||
|---|---|---|---|---|---|---|
| γ-Al2O3 | Amorphous alumina | γ-Al2O3 | Amorphous alumina | γ-Al2O3 | Amorphous alumina | |
| 0.01 | 3.35×10-3 | 2.69×10-3 | - | - | 4.52 | 4.25 |
| 0.10 | 2.94×10-3 | 1.79×10-3 | - | - | ||
| 0.70 | 2.75×10-3 | 1.09×10-3 | -0.809 | -1.380 | ||
Table 1 Surface acid-base properties of two aluminum oxides in different ionic strength media
| NaNO3 concentration/ (mol·L-1) | HS concentration/(mol·L-1) | TOTHpH=8/(mmol·L-1) | pHPZNPC* | |||
|---|---|---|---|---|---|---|
| γ-Al2O3 | Amorphous alumina | γ-Al2O3 | Amorphous alumina | γ-Al2O3 | Amorphous alumina | |
| 0.01 | 3.35×10-3 | 2.69×10-3 | - | - | 4.52 | 4.25 |
| 0.10 | 2.94×10-3 | 1.79×10-3 | - | - | ||
| 0.70 | 2.75×10-3 | 1.09×10-3 | -0.809 | -1.380 | ||
| Medium | Frap | Fslow | kslow/h-1 | krap/h-1 | Qe/(mg·g-1) | r2 |
|---|---|---|---|---|---|---|
| 0.7 mol·L-1 NaNO3 | 0.813 | 0.187 | 0.977 | 26.32 | 21.42 | 0.977 |
| NSW | 0.740 | 0.260 | 0.193 | 20.23 | 16.56 | 0.988 |
Table 2 Sorption kinetic parameters of phosphorus on amorphous alumina
| Medium | Frap | Fslow | kslow/h-1 | krap/h-1 | Qe/(mg·g-1) | r2 |
|---|---|---|---|---|---|---|
| 0.7 mol·L-1 NaNO3 | 0.813 | 0.187 | 0.977 | 26.32 | 21.42 | 0.977 |
| NSW | 0.740 | 0.260 | 0.193 | 20.23 | 16.56 | 0.988 |
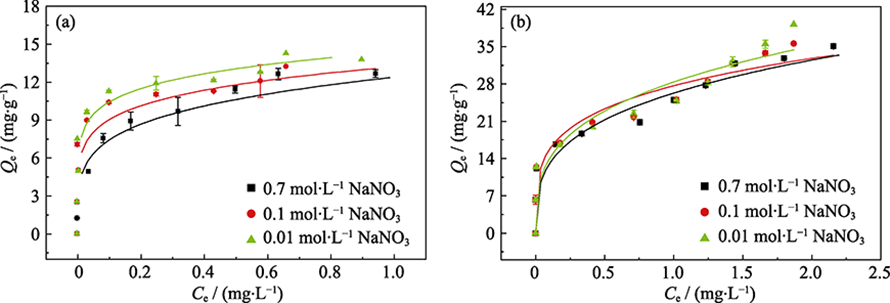
Fig. 5 Sorption isotherms of phosphate on γ-Al2O3 (a) and amorphous alumina (b) in NaNO3 with different concentrations Dot: experimental data; Line: Freundlich model fitting
| Sorbent | I/(mol·L-1) | Freundlich | Langmuir | ||||
|---|---|---|---|---|---|---|---|
| KF/(mg·g-1) (L·mg-1)1/n | n | r2 | Qm/(mg·g-1) | KL/(L·mg-1) | r2 | ||
| γ-Al2O3 | 0.7 | 12.39 | 4.52 | 0.942 | 14.93 | 5.288 | 0.848 |
| 0.1 | 13.20 | 6.08 | 0.912 | 13.47 | 18.29 | 0.702 | |
| 0.01 | 14.37 | 7.20 | 0.896 | 14.28 | 27.90 | 0.485 | |
| Amorphous alumina | 0.7 | 26.28 | 3.27 | 0.933 | 34.70 | 3.828 | 0.838 |
| 0.1 | 27.66 | 4.09 | 0.933 | 34.60 | 4.888 | 0.820 | |
| 0.01 | 28.36 | 3.24 | 0.907 | 43.22 | 2.046 | 0.817 | |
Table 3 Isothermal sorption parameters of phosphates on γ-Al2O3 and amorphous alumina
| Sorbent | I/(mol·L-1) | Freundlich | Langmuir | ||||
|---|---|---|---|---|---|---|---|
| KF/(mg·g-1) (L·mg-1)1/n | n | r2 | Qm/(mg·g-1) | KL/(L·mg-1) | r2 | ||
| γ-Al2O3 | 0.7 | 12.39 | 4.52 | 0.942 | 14.93 | 5.288 | 0.848 |
| 0.1 | 13.20 | 6.08 | 0.912 | 13.47 | 18.29 | 0.702 | |
| 0.01 | 14.37 | 7.20 | 0.896 | 14.28 | 27.90 | 0.485 | |
| Amorphous alumina | 0.7 | 26.28 | 3.27 | 0.933 | 34.70 | 3.828 | 0.838 |
| 0.1 | 27.66 | 4.09 | 0.933 | 34.60 | 4.888 | 0.820 | |
| 0.01 | 28.36 | 3.24 | 0.907 | 43.22 | 2.046 | 0.817 | |
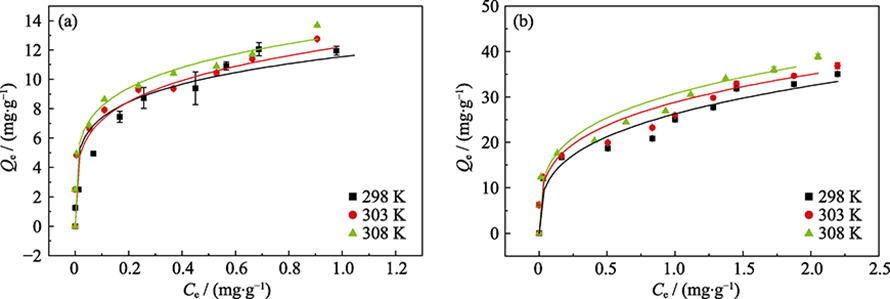
Fig. 6 Sorption isotherms of phosphate on γ-Al2O3 (a) and amorphous alumina (b) at different femperatures Dot: experimental data; Line: Freundlich model fitting
| Adsorbent | T/K | KF/(mg·g-1) (L·mg-1)1/n | n | r2 | ΔGθ/(kJ·mol-1) | ΔHθ/(kJ·mol-1) | ΔSθ/(J·mol-1·K-1) |
|---|---|---|---|---|---|---|---|
| γ-Al2O3 | 298 | 11.55 | 5.18 | 0.938 | -23.18 | 9.007 | 108.0 |
| 303 | 12.22 | 4.40 | 0.948 | -23.71 | |||
| 308 | 12.99 | 4.98 | 0.952 | -24.26 | |||
| Amorphous alumina | 298 | 26.28 | 3.27 | 0.933 | -25.21 | 11.96 | 124.8 |
| 303 | 28.84 | 3.60 | 0.947 | -25.87 | |||
| 308 | 30.73 | 3.64 | 0.951 | -26.46 |
Table 4 Sorption thermodynamic parameters of phosphate on γ-Al2O3 and amorphous alumina
| Adsorbent | T/K | KF/(mg·g-1) (L·mg-1)1/n | n | r2 | ΔGθ/(kJ·mol-1) | ΔHθ/(kJ·mol-1) | ΔSθ/(J·mol-1·K-1) |
|---|---|---|---|---|---|---|---|
| γ-Al2O3 | 298 | 11.55 | 5.18 | 0.938 | -23.18 | 9.007 | 108.0 |
| 303 | 12.22 | 4.40 | 0.948 | -23.71 | |||
| 308 | 12.99 | 4.98 | 0.952 | -24.26 | |||
| Amorphous alumina | 298 | 26.28 | 3.27 | 0.933 | -25.21 | 11.96 | 124.8 |
| 303 | 28.84 | 3.60 | 0.947 | -25.87 | |||
| 308 | 30.73 | 3.64 | 0.951 | -26.46 |
| [1] |
JALALI M, PEIKAM E N. Phosphorus sorption-desorption behaviour of river bed sediments in the Abshineh river, Hamedan, Iran, related to their composition. Environmental Monitoring and Assessment, 2013,185(1):537-552.
DOI URL PMID |
| [2] |
COTOVICZ JUNIOR L C, MACHADO E D, BRANDINI N,et al. Distributions of total, inorganic and organic phosphorus in surface and recent sediments of the sub-tropical and semi-pristine Guaratuba Bay estuary, SE Brazil. Environmental Earth Sciences, 2014,72(2):373-386.
DOI URL |
| [3] |
HUANG S, HUANG H, ZHU H. Effects of the addition of iron and aluminum salt on phosphorus adsorption in wetland sediment. Environmental Science and Pollution Research, 2016,23(10):10022-10027.
URL PMID |
| [4] |
QIAN L, MA M, CHENG D. The effect of water chemistry on adsorption and desorption of U(VI) on nano-alumina. Journal of Molecular Liquids, 2014,197:295-300.
DOI URL |
| [5] | CUI Y, XIAO R, XIE Y,et al. Phosphorus fraction and phosphate sorption-release characteristics of the wetland sediments in the Yellow River Delta. Physics and Chemistry of the Earth, 2018,103:19-27. |
| [6] | 王赫, 贾永锋, 刘利. 沉积物中典型氧化物矿物吸附的镉对芦苇的生物有效性研究. 环境科学, 2009,30(6):215-220. |
| [7] |
LAING G D, RINKLEBE J, VANDECASTEELE B,et al. Trace metal behaviour in estuarine and riverine floodplain soils and sediments: a review. Science of the Total Environment, 2009,407(13):3972-3985.
URL PMID |
| [8] | 杨晓芳, 王东升, 孙中溪. 三水铝石(γ-Al(OH)3)和α-Al2O3表面酸碱性质与磷酸根吸附研究. 环境科学学报, 2007,27(4):637-642. |
| [9] | 孟文娜, 谢杰, 吴德意. 活性氧化铝对水中磷的去除与回收研究. 环境科学, 2013,34(1):231-236. |
| [10] |
KIM Y, KIRKPATRICK R J. An investigation of phosphate adsorbed on aluminium oxyhydroxide and oxide phases by nuclear magnetic resonance. European Journal of Soil Science, 2004,55(2):243-251.
DOI URL |
| [11] |
JOHNSON B B, IVANOV A V, ANTZUTKIN O N,et al. 31P nuclear magnetic resonance study of the adsorption of phosphate and phenyl phosphates on γ-Al2O3. Langmuir, 2002,18(4):1104-1111.
DOI URL |
| [12] | 杨晓芳. 原位红外光谱研究含氧离子氧化物微界面吸附过程与机理. 北京: 中国科学院大学博士学位论文, 2010. |
| [13] |
邵兴华, 章永松, 林咸永. 三种铁氧化物的磷吸附解吸特性以及与磷吸附饱和度的关系. 植物营养与肥料学报, 2006,12(2):208-212.
DOI URL |
| [14] | CHATMAN S, ZARZYCKI P, PREOANIN T,et al. Effect of surface site interactions on potentiometric titration of hematite (α-Fe2O3) crystal faces. Journal of Colloid and Interface Science, 2013,39(1):125-134. |
| [15] | 严玉鹏. 几种土壤有机磷在铁铝氧化物表面的吸附、解吸与沉淀. 武汉: 华中农业大学硕士学位论文, 2015. |
| [16] | 杜淼, 李斌, 任海萍, 等. 纳米介孔氧化铝制备及表征. 无机盐工业, 2008,40(5):34-35. |
| [17] | DIZ P, MENA A, NOMBELA MIGUEL ÁNGEL, et al. Description of an experimental set up for the culture of benthic foraminifera in controlled pH conditions. Thalassas: An International Journal of Marine Sciences, 2015,31(1):23-32. |
| [18] | 张学清, 项金钟, 胡永茂. 纳米Al2O3的制备及红外吸收研究. 中国陶瓷, 2004,40(1):24-27. |
| [19] |
AZIZIAN S. Kinetic models of sorption: a theoretical analysis. Journal of Colloid and Interface Science, 2004,276(1):47-52.
DOI URL PMID |
| [20] | 李显波, 马力, 刘志红, 等. 活性氧化铝对废水中磷酸根离子的吸附特性研究. 非金属矿, 2017,40(4):4-7. |
| [21] | 张炳慧. 天然水中磷酸盐存在状态与pH值的关系. 地质实验室, 1992,8(2):98-100. |
| [22] | CHUBAR N I, KANIBOLOTSKYY V A, STRELKO V V,et al. Adsorption of phosphate ions on novel inorganic ion exchangers. Colloids and Surfaces A (Physicochemical and Engineering Aspects), 2005,255(1/2/3):55-63. |
| [1] | TUERHONG Munire, ZHAO Honggang, MA Yuhua, QI Xianhui, LI Yuchen, YAN Chenxiang, LI Jiawen, CHEN Ping. Construction and Photocatalytic Activity of Monoclinic Tungsten Oxide/Red Phosphorus Step-scheme Heterojunction [J]. Journal of Inorganic Materials, 2023, 38(6): 701-707. |
| [2] | SUN Qiangqiang, CHEN Zixuan, YANG Ziyue, WANG Yimeng, CAO Baoyue. Amorphous Vanadium Oxide Loaded by Metallic Nickel-copper towards High-efficiency Electrocatalyzing Hydrogen Production [J]. Journal of Inorganic Materials, 2023, 38(6): 647-655. |
| [3] | WU Rui, ZHANG Minhui, JIN Chenyun, LIN Jian, WANG Deping. Photothermal Core-Shell TiN@Borosilicate Bioglass Nanoparticles: Degradation and Mineralization [J]. Journal of Inorganic Materials, 2023, 38(6): 708-716. |
| [4] | WANG Shiyi, FENG Aihu, LI Xiaoyan, YU Yun. Pb (II) Adsorption Process of Fe3O4 Supported Ti3C2Tx [J]. Journal of Inorganic Materials, 2023, 38(5): 521-528. |
| [5] | WU Shuang, GOU Yanzi, WANG Yongshou, SONG Quzhi, ZHANG Qingyu, WANG Yingde. Effect of Heat Treatment on Composition, Microstructure and Mechanical Property of Domestic KD-SA SiC Fibers [J]. Journal of Inorganic Materials, 2023, 38(5): 569-576. |
| [6] | GUO Chunxia, CHEN Weidong, YAN Shufang, ZHAO Xueping, YANG Ao, MA Wen. Adsorption of Arsenate in Water by Zirconia-halloysite Nanotube Material [J]. Journal of Inorganic Materials, 2023, 38(5): 529-536. |
| [7] | MA Xiaosen, ZHANG Lichen, LIU Yanchao, WANG Quanhua, ZHENG Jiajun, LI Ruifeng. 13X@SiO2: Synthesis and Toluene Adsorption [J]. Journal of Inorganic Materials, 2023, 38(5): 537-543. |
| [8] | HE Danqi, WEI Mingxu, LIU Ruizhi, TANG Zhixin, ZHAI Pengcheng, ZHAO Wenyu. Heavy-Fermion YbAl3 Materials: One-step Synthesis and Enhanced Thermoelectric Performance [J]. Journal of Inorganic Materials, 2023, 38(5): 577-582. |
| [9] | ZHANG Wanwen, LUO Jianqiang, LIU Shujuan, MA Jianguo, ZHANG Xiaoping, YANG Songwang. Zirconia Spacer: Preparation by Low Temperature Spray-coating and Application in Triple-layer Perovskite Solar Cells [J]. Journal of Inorganic Materials, 2023, 38(2): 213-218. |
| [10] | YU Yefan, XU Ling, NI Zhongbing, SHI Dongjian, CHEN Mingqing. Prussian Blue Modified Biochar: Preparation and Adsorption of Ammonia Nitrogen from Sewage [J]. Journal of Inorganic Materials, 2023, 38(2): 205-212. |
| [11] | WANG Hongning, HUANG Li, QING Jiang, MA Tengzhou, HUANG Weiqiu, CHEN Ruoyu. Mesoporous Organic-inorganic Hybrid Siliceous Hollow Spheres: Synthesis and VOCs Adsorption [J]. Journal of Inorganic Materials, 2022, 37(9): 991-1000. |
| [12] | LIU Qi, ZHU Can, XIE Guizhen, WANG Jun, ZHANG Dongming, SHAO Gangqin. Optical Absorption and Photoluminescence Spectra of Ce-doped SrMgF4 Polycrystalline with Superlattice Structure [J]. Journal of Inorganic Materials, 2022, 37(8): 897-902. |
| [13] | ZHANG Ye, ZENG Yuping. Progress of Porous Silicon Nitride Ceramics Prepared via Self-propagating High Temperature Synthesis [J]. Journal of Inorganic Materials, 2022, 37(8): 853-864. |
| [14] | CHENG Weijie, WANG Minglei, LIN Guoqiang. Composition, Structure and Properties of CrAlN-DLC Hard Composite Films Deposited by Arc Ion Plating [J]. Journal of Inorganic Materials, 2022, 37(7): 764-772. |
| [15] | WEN Zhiqin, HUANG Binrong, LU Taoyi, ZOU Zhengguang. Pressure on the Structure and Thermal Properties of PbTiO3: First-principle Study [J]. Journal of Inorganic Materials, 2022, 37(7): 787-794. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||