
Journal of Inorganic Materials ›› 2020, Vol. 35 ›› Issue (7): 769-780.DOI: 10.15541/jim20190433
Special Issue: 能源材料论文精选(二):超级电容器与储能电池(2020); 【虚拟专辑】超级电容器(2020~2021)
• REVIEW • Previous Articles Next Articles
LI Zehui1,TAN Meijuan2,ZHENG Yuanhao3,LUO Yuyang3,JING Qiushi3,JIANG Jingkun1,LI Mingjie4( )
)
Received:2019-08-16
Revised:2019-09-29
Published:2020-07-20
Online:2019-10-23
Supported by:CLC Number:
LI Zehui,TAN Meijuan,ZHENG Yuanhao,LUO Yuyang,JING Qiushi,JIANG Jingkun,LI Mingjie. Application of Conductive Metal Organic Frameworks in Supercapacitors[J]. Journal of Inorganic Materials, 2020, 35(7): 769-780.
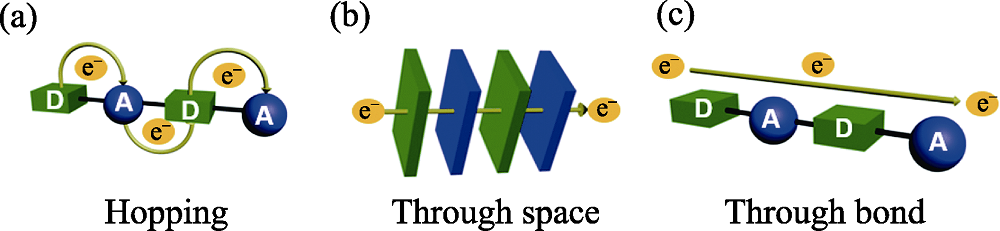
Fig. 1 Representation of possible modes of charge transport in MOFs: (a) hopping charge transport, (b) through-space charge transport, and (c) through-bond charge transport[22]
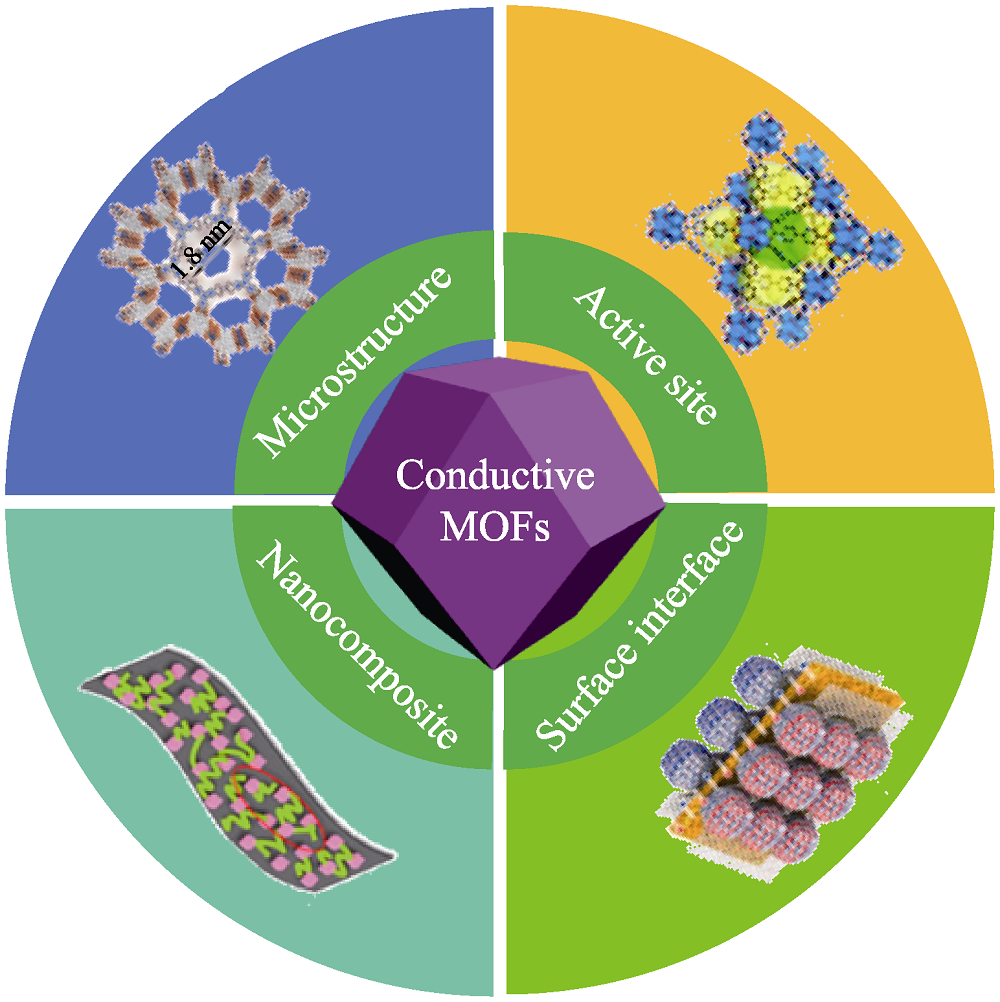
Fig. 2 Control strategy of conductive MOFs in supercapacitors From microstructure, active site, surface interface and nanocomposite of conductive MOFs to energy storage applications
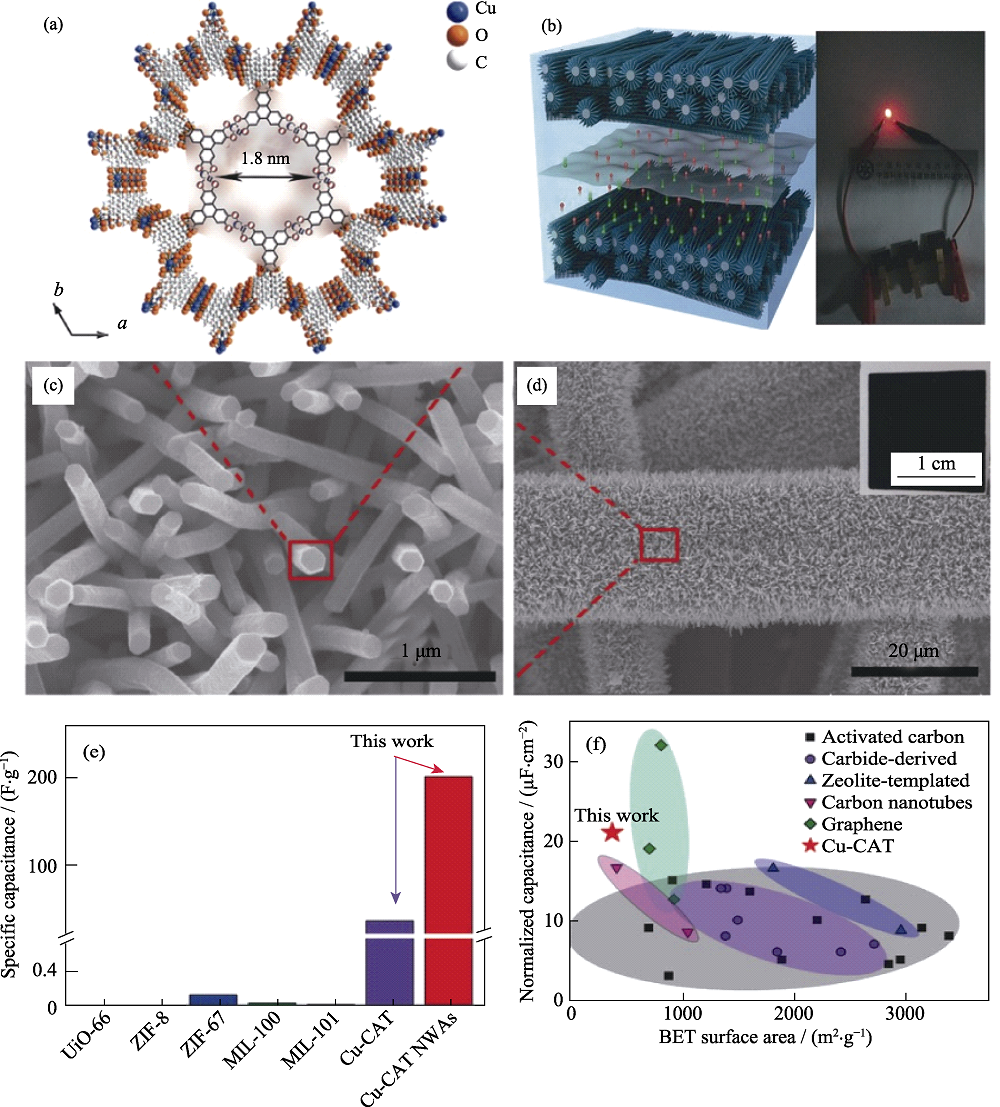
Fig. 3 (a) Crystal structure of Cu-CAT viewed along the c-axis; (b) Structure of the solid-state supercapacitor (left) and photograph of a red light-emitting-diode powered by the three supercapacitors connected in series (right); (c, d) SEM and photographic images (inset in (d)) of the Cu-CAT NWAs growing on carbon fiber paper; Performance comparison of (e)Cu-CAT NWAs and (f) MOF materials, and carbon materials based symmetric solid-state supercapacitors[29]
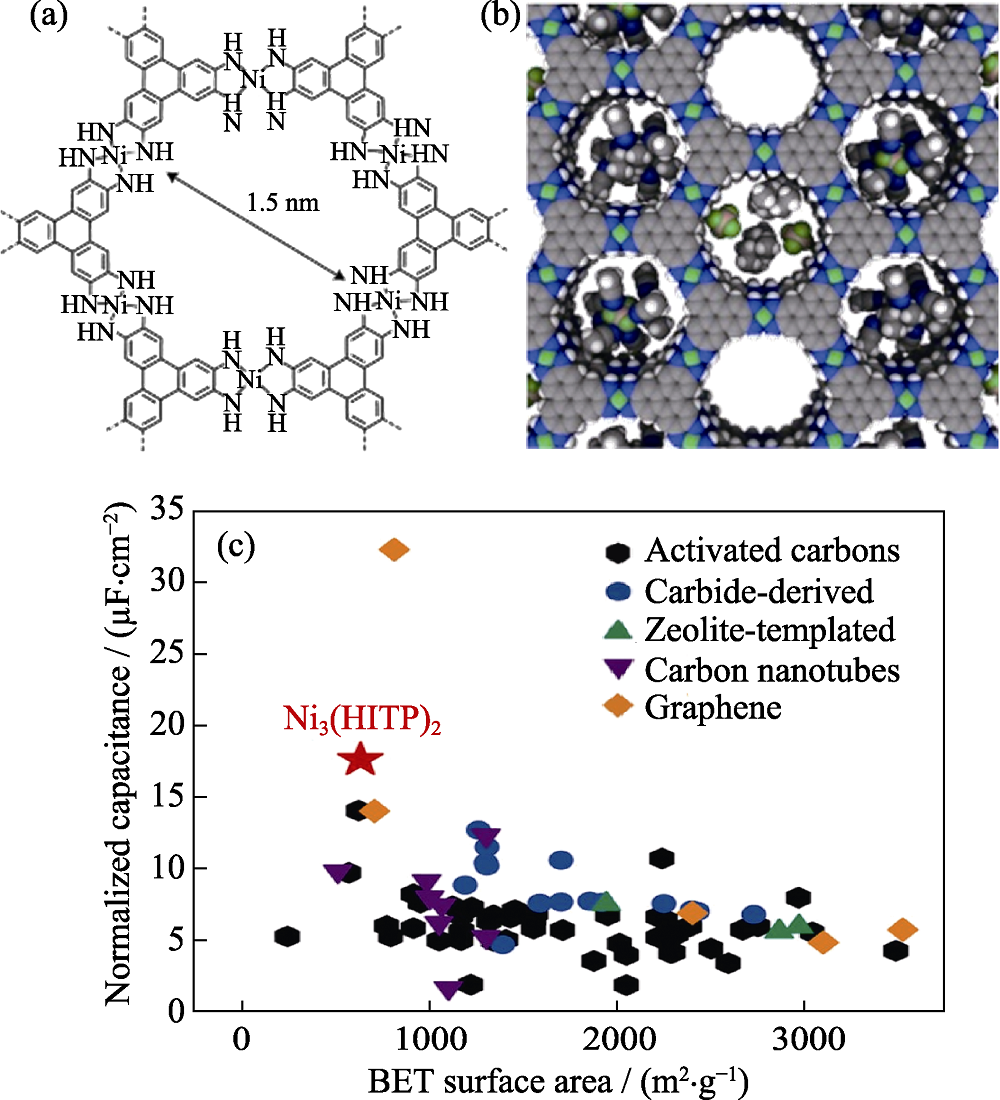
Fig. 4 (a) Molecular structure of Ni3(HITP)2; (b) Relative size of pores, electrolyte Et4N+ and BF4- ions, and acetonitrile solvent molecules showing in a space-filling diagram of idealized Ni3(HITP)2; (c) Comparison of areal capacitance for various materials normalized relative to their BET surface areas[30]
| Metal | Organic ligand | MOFs | Conductivity /(S?m-1) | SBET /(m2·g-1) | Specific capacitance | Energy density | Power density | Ref. |
|---|---|---|---|---|---|---|---|---|
| Fe | 1,3,5-Benzenetricarboxylic acid (H3BT)C | MIL-100 | - | - | 39 F·g-1 | - | - | [ |
| Co | Polyethylene glycol (PEG) | Co-MOF-71 | - | - | 206.76 F·g-1 | 7.18 Wh·kg-1 | - | [ |
| Co | Benzendicarboxylic (BDC) acid | Co-BDC | - | 9.09 | 131.8 F·g-1 | 20.7 Wh·kg-1 | 3880 W·kg-1 | [ |
| Co | 2,6-Naphthalenedicarboxylic (NDC) acid | Co-NDC | - | 20.29 | 147.3 F·g-1 | 23.1 Wh·kg-1 | 5490 W·kg-1 | [ |
| Co | 4,4-Biphenyldicarboxylic (BPDC) acid | Co-BPDC | - | 138.35 | 179.2 F·g-1 | 31.4 Wh·kg-1 | 5640 W·kg-1 | [ |
| Ni | Isonicotinic acid | Ni-MOF | - | 148 | 634 F·g-1 | - | - | [ |
| Ni | Salicylate ion | 1D Ni-MOF | - | 186.8 | 1698 F·g-1 | - | - | [ |
| Ni | p-Benzenedicarboxylic acid (PTA) | Ni-MOF-24 | - | - | 1127 F·g-1 | 19.17 Wh·kg-1 | 1750 W·kg-1 | [ |
| Ni | 1,3,5-Benzenetricarboxylic (btc) acid (H3BT)C | Ni3(btc)2·12H2O | - | - | 726 F·g-1 | 16.5 Wh·kg-1 | 2078 W·kg-1 | [ |
| Ni | 9,10-Anthracenedicarboxylic acid (ADC) | Ni-DMOF-ADC | - | 783 | 552 F·g-1 | - | - | [ |
| Ni | 2,3,6,7,10,11-Hexaiminotriphenylene (HITP) | Ni3(HITP)2 | >5000 | 630 | 111 F·g-1 | - | - | [ |
| Zn | 4,4’-Biphenyldicarboxylic acid (H2BPC) | Zn6(BPC)6(L)3· 9DMF | - | - | 23 F·g-1 | 1.9 Wh·kg-1 | 3 W·kg-1 | [ |
| Zn | p-Phenylenediamine (pPDA) | Zn-(pPDA)MOF | 0.1~1.05 | 200.86 F·g-1 | 62.8 Wh·kg-1 | 4500 W·kg-1 | [ | |
| Cd | 2,5-Thiophenedicarboxylic acid (H2TDC) | Cd2(TDC)2(L)2· 2H2O | - | - | 22 F·g-1 | 2.1 Wh·kg-1 | 3.3 W·kg-1 | [ |
| Zr | 2,2’-Bipyridine-5,5’-dicarboxylate (BPYDC) | nMOF-867 | - | - | 5.085 mF·cm-2 | 6.04× 10-4 Wh?cm-3stack | 1.097 W·cm-3stack | [ |
| Zn, Ni | p-Benzenedicarboxylic acid (PTA) | Zn-doped Ni-MOF | - | - | 1620 F·g-1 | 27.56 Wh·kg-1 | 1750 W·kg-1 | [ |
| Co, Zn | Benzendicarboxylic acid | Co8-MOF-5 | - | 2900 | 0.49 F·g-1 | - | - | [ |
| Zn, Zr | Terephthalic acid | HP-UiO-66 | - | - | 849 F·g-1 | 32 Wh·kg-1 | 240 W·kg-1 | [ |
Table 1 MOFs with different center metal atoms for SCs
| Metal | Organic ligand | MOFs | Conductivity /(S?m-1) | SBET /(m2·g-1) | Specific capacitance | Energy density | Power density | Ref. |
|---|---|---|---|---|---|---|---|---|
| Fe | 1,3,5-Benzenetricarboxylic acid (H3BT)C | MIL-100 | - | - | 39 F·g-1 | - | - | [ |
| Co | Polyethylene glycol (PEG) | Co-MOF-71 | - | - | 206.76 F·g-1 | 7.18 Wh·kg-1 | - | [ |
| Co | Benzendicarboxylic (BDC) acid | Co-BDC | - | 9.09 | 131.8 F·g-1 | 20.7 Wh·kg-1 | 3880 W·kg-1 | [ |
| Co | 2,6-Naphthalenedicarboxylic (NDC) acid | Co-NDC | - | 20.29 | 147.3 F·g-1 | 23.1 Wh·kg-1 | 5490 W·kg-1 | [ |
| Co | 4,4-Biphenyldicarboxylic (BPDC) acid | Co-BPDC | - | 138.35 | 179.2 F·g-1 | 31.4 Wh·kg-1 | 5640 W·kg-1 | [ |
| Ni | Isonicotinic acid | Ni-MOF | - | 148 | 634 F·g-1 | - | - | [ |
| Ni | Salicylate ion | 1D Ni-MOF | - | 186.8 | 1698 F·g-1 | - | - | [ |
| Ni | p-Benzenedicarboxylic acid (PTA) | Ni-MOF-24 | - | - | 1127 F·g-1 | 19.17 Wh·kg-1 | 1750 W·kg-1 | [ |
| Ni | 1,3,5-Benzenetricarboxylic (btc) acid (H3BT)C | Ni3(btc)2·12H2O | - | - | 726 F·g-1 | 16.5 Wh·kg-1 | 2078 W·kg-1 | [ |
| Ni | 9,10-Anthracenedicarboxylic acid (ADC) | Ni-DMOF-ADC | - | 783 | 552 F·g-1 | - | - | [ |
| Ni | 2,3,6,7,10,11-Hexaiminotriphenylene (HITP) | Ni3(HITP)2 | >5000 | 630 | 111 F·g-1 | - | - | [ |
| Zn | 4,4’-Biphenyldicarboxylic acid (H2BPC) | Zn6(BPC)6(L)3· 9DMF | - | - | 23 F·g-1 | 1.9 Wh·kg-1 | 3 W·kg-1 | [ |
| Zn | p-Phenylenediamine (pPDA) | Zn-(pPDA)MOF | 0.1~1.05 | 200.86 F·g-1 | 62.8 Wh·kg-1 | 4500 W·kg-1 | [ | |
| Cd | 2,5-Thiophenedicarboxylic acid (H2TDC) | Cd2(TDC)2(L)2· 2H2O | - | - | 22 F·g-1 | 2.1 Wh·kg-1 | 3.3 W·kg-1 | [ |
| Zr | 2,2’-Bipyridine-5,5’-dicarboxylate (BPYDC) | nMOF-867 | - | - | 5.085 mF·cm-2 | 6.04× 10-4 Wh?cm-3stack | 1.097 W·cm-3stack | [ |
| Zn, Ni | p-Benzenedicarboxylic acid (PTA) | Zn-doped Ni-MOF | - | - | 1620 F·g-1 | 27.56 Wh·kg-1 | 1750 W·kg-1 | [ |
| Co, Zn | Benzendicarboxylic acid | Co8-MOF-5 | - | 2900 | 0.49 F·g-1 | - | - | [ |
| Zn, Zr | Terephthalic acid | HP-UiO-66 | - | - | 849 F·g-1 | 32 Wh·kg-1 | 240 W·kg-1 | [ |
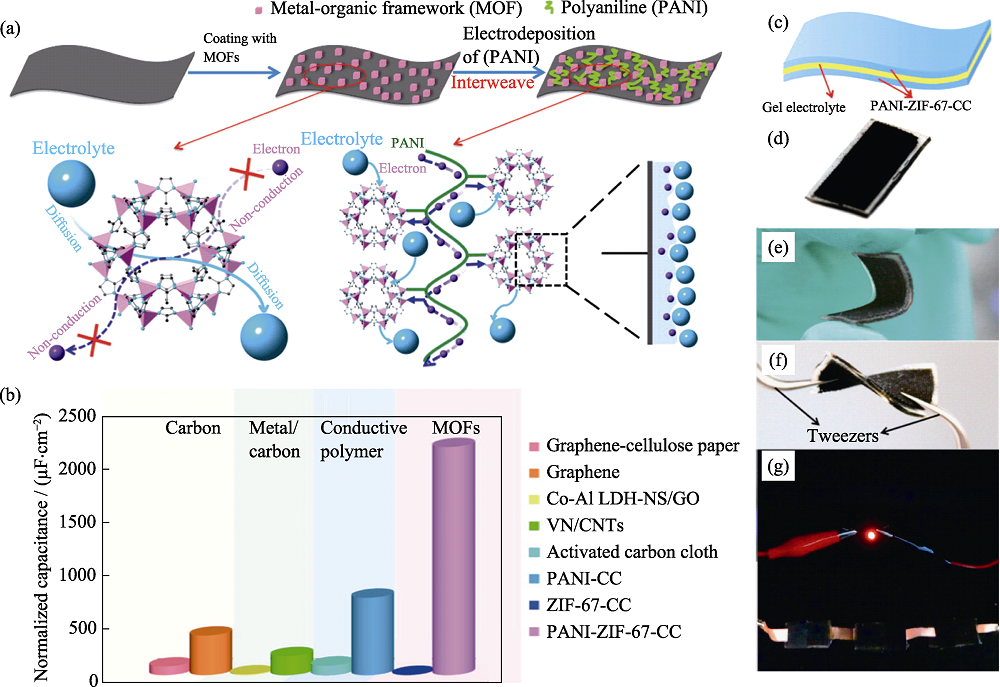
Fig. 8 (a) Illustration of the synthesis methodology for polyaniline-ZIF-67 on carbon cloth; (b) Areal capacitances of the PANI-ZIF-67-CC and other electrode materials; (c-f) Schematic illustrations of PANI-ZIF-67-CC flexible solid-state SC device; (g) Photograph of a red light-emitting-diode (LED) powered by the three SCs connected in series[52]
| [1] |
MEHTAB T, YASIN G, ARIF M, et al. Metal-organic frameworks for energy storage devices: batteries and supercapacitors. J. Energy Storage, 2019,21:632-646.
DOI URL |
| [2] |
FAN Z, YAN J, TONG W, et al. Asymmetric supercapacitors based on graphene/MnO2 and activated carbon nanofiber electrodes with high power and energy density. Adv. Funct. Mater., 2011,21(12):2366-2375.
DOI URL |
| [3] |
CHEN W, YU H, LEE S Y, et al. Nanocellulose: a promising nanomaterial for advanced electrochemical energy storage. Chem. Soc. Rev., 2018,47(8):2837-2872.
DOI URL PMID |
| [4] |
CHENG J, CHEN S, CHEN D, et al. Editable asymmetric all-solid-state supercapacitors based on high-strength, flexible, and programmable 2D-metal-organic framework/reduced graphene oxide self-assembled papers. J. Mater. Chem. A, 2018,6(41):20254-20266.
DOI URL |
| [5] |
XUAN W, ZHU C, LIU Y, et al. Mesoporous metal-organic framework materials. Chem. Soc. Rev., 2012,41(5):1677-1695.
DOI URL PMID |
| [6] |
ZHU Q L, XU Q. Metal-organic framework composites. Chem. Soc. Rev., 2014,43(16):5468-5512.
DOI URL PMID |
| [7] |
ZHENG S, LI X, YAN B, et al. Transition-metal (Fe, Co, Ni) based metal-organic frameworks for electrochemical energy storage. Adv. Energy Mater., 2017,7(18):1602733.
DOI URL |
| [8] |
JIANG L, SHENG L, LONG C, et al. Densely packed graphene nanomesh-carbon nanotube hybrid film for ultra-high volumetric performance supercapacitors. Nano Energy, 2015,11(21):471-480.
DOI URL |
| [9] |
SUNDRIYAL S, KAUR H, BHARDWAJ S K, et al. Metal-organic frameworks and their composites as efficient electrodes for supercapacitor applications. Coord. Chem. Rev., 2018,369:15-38.
DOI URL |
| [10] | LI L X, TAO J, GENG X, et al. Preparation and supercapacitor performance of nitrogen-doped carbon nanotubes from polyaniline modification. Acta Phys-Chim. Sin., 2013,29(1):924-929. |
| [11] |
YAN J, TONG W, BO S, et al. Preparation of a graphene nanosheet/ polyaniline composite with high specific capacitance. Carbon, 2010,48(2):487-493.
DOI URL |
| [12] | SUBRAMANIAN V, ZHU H, VAJTAI R, et al. Hydrothermal synthesis and pseudocapacitance properties of MnO2 nanostructures. J. Physi. Chem. B, 2005,109(43):20207-20214. |
| [13] |
WANG Q, WEN Z, JINGHONG L. A hybrid supercapacitor fabricated with a carbon nanotube cathode and a TiO2-B nanowire anode. Adv. Funct. Mater., 2010,16:2141-2146.
DOI URL |
| [14] |
ROWSELL J L C, YAGHI O M. Metal-organic frameworks: a new class of porous materials. Micropor. Mesopor. Mat., 2004,73(1):3-14.
DOI URL |
| [15] |
HENDON C H, TIANA D, WALSH A. Conductive metal-organic frameworks and networks: fact or fantasy? Phys. Chem. Chem. Phys., 2012,14(38):13120-13132.
DOI URL PMID |
| [16] |
KOBAYASHI Y, JACOBS B, ALLENDORF M D, et al. Conductivity, doping, and redox chemistry of a microporous dithiolene-based metal-organic framework. Chem. Mater., 2010,22(14):4120-4122.
DOI URL |
| [17] |
GÁNDARA F, URIBE-ROMO F J, BRITT D K, et al. Porous, conductive metal-triazolates and their structural elucidation by the charge-flipping method. Chem. Eur-J., 2012,18(34):10595-10601.
DOI URL PMID |
| [18] |
LI R, WANG S H, CHEN X X, et al. Highly anisotropic and water molecule-dependent proton conductivity in a 2D homochiral copper (II) metal-organic framework. Chem. Mater., 2017,29(5):2321-2331.
DOI URL |
| [19] |
SADAKIYO M, YAMADA T, KITAGAWA H. Rational designs for highly proton-conductive metal-organic frameworks. J. Am. Chem. Soc., 2009,131(29):9906-9007.
DOI URL PMID |
| [20] |
SADAKIYO M, YAMADA T, KITAGAWA H. Proton conductivity control by ion substitution in a highly proton-conductive metal-organic framework. J. Am. Chem. Soc., 2014,136(38):13166-13169.
DOI URL |
| [21] |
TAYLOR J M, DEKURA S, IKEDA R, et al. Defect control to enhance proton conductivity in a metal-organic framework. Chem. Mater., 2015,27(7):2286-2289.
DOI URL |
| [22] |
KO M, MENDECKI L, MIRICA K A. Conductive two-dimensional metal-organic frameworks as multifunctional materials. Chem. Commun., 2018,54(57):7873-7891.
DOI URL |
| [23] |
SUN L, HENDON C H, MINIER M A, et al. Million-fold electrical conductivity enhancement in Fe2(DEBDC) versus Mn2(DEBDC) (E=S, O). J. Am. Chem. Soc., 2015,137(19):6164-6167.
DOI URL PMID |
| [24] |
LIN S, USOV P M, MORRIS A J. The role of redox hopping in metal-organic framework electrocatalysis. Chem. Commun., 2018,54(51):6965-6974.
DOI URL |
| [25] |
LEE D Y, SHINDE D V, KIM E K, et al. Supercapacitive property of metal-organic-frameworks with different pore dimensions and morphology. Micropor. Mesopor. Mat., 2013,171(10):53-57.
DOI URL |
| [26] |
HUANG H, LI J R, WANG K, et al. An in situ self-assembly template strategy for the preparation of hierarchical-pore metal-organic frameworks. Nat. Commun., 2015,6:8847.
DOI URL PMID |
| [27] |
HOU J, CAO C, IDREES F, et al. Hierarchical porous nitrogen-doped carbon nanosheets derived from silk for ultrahigh-capacity battery anodes and supercapacitors. ACS Nano, 2015,9(3):2556-2564.
DOI URL PMID |
| [28] |
TAN Y, ZHANG W, GAO Y, et al. Facile synthesis and supercapacitive properties of Zr-metal organic frameworks (UiO-66). RSC Adv., 2015,5(23):17601-17605.
DOI URL |
| [29] |
LI W H, KUI D, TIAN H R, et al. Conductive metal-organic framework nanowire array electrodes for high-performance solid-state supercapacitors. Adv. Funct. Mater., 2017,27(27):1702067.
DOI URL |
| [30] |
SHEBERLA D, BACHMAN J C, ELIAS J S, et al. Conductive MOF electrodes for stable supercapacitors with high areal capacitance. Nat. Mater., 2017,16(2):220-224.
DOI URL PMID |
| [31] |
WEI C, RAKHI R B, WANG Q, et al. Morphological and electrochemical cycling effects in MnO2 nanostructures by 3D electron tomography. Adv. Funct. Mater., 2014,24(21):3130-3143.
DOI URL |
| [32] |
CHEN Y, DAN N, YANG X, et al. Microwave-assisted synthesis of honeycomblike hierarchical spherical Zn-doped Ni-MOF as a high-performance battery-type supercapacitor electrode material. Electrochim. Acta, 2018,278:114-123.
DOI URL |
| [33] |
ZHANG J, HAN B. Supercritical or compressed CO2 as a stimulus for tuning surfactant aggregations. Accounts Chem. Res., 2013,46(2):425-433.
DOI URL |
| [34] |
YU H, XU D, XU Q. Dual template effect of supercritical CO2 in ionic liquid to fabricate a highly mesoporous cobalt metal-organic framework. Chem. Commun., 2015,51(67):13197-13200.
DOI URL |
| [35] |
CAMPAGNOL N, ROMERO-VARA R, DELEU W, et al. A hybrid supercapacitor based on porous carbon and the metal-organic framework MIL-100(Fe). ChemElectroChem, 2014,1(7):1182-1188.
DOI URL |
| [36] |
LEE D Y, YOON S J, SHRESTHA N K, et al. Unusual energy storage and charge retention in Co-based metal-organic-frameworks. Micropor. Mesopor. Mat., 2012,153(3):163-165.
DOI URL |
| [37] | LIAO C, ZUO Y, WEI Z, et al. Russ. Electrochemical performance of metal-organic framework synthesized by a solvothermal method for supercapacitors. J. Electrochem., 2013,49(10):983-986. |
| [38] | XU J, CHAO Y, XUE Y, et al. Facile synthesis of novel metal-organic nickel hydroxide nanorods for high performance supercapacitor. Electrochim. Acta, 2016,211:595-602. |
| [39] | YANG J, XIONG P, ZHENG C, et al. Metal-organic frameworks: a new promising class of material for high performances supercapacitor electrode. J. Mater. Chem. A, 2014,2(39):16640-16644. |
| [40] | KANG L, SUN S X, KONG L B, et al. Investigating metal-organic framework as a new pseudo-capacitive material for supercapacitors. Chinese Chem. Lett., 2014,25(6):957-961. |
| [41] | QU C, JIAO Y, ZHAO B, et al. Nickel-based pillared MOFs for high-performance supercapacitors: design, synthesis and stability study. Nano Energy, 2016,26:66-73. |
| [42] |
GONG Y, LI J, JIANG P, et al. Novel metal(II) coordination polymers based on N,N'-bis-(4-pyridyl)phthalamide as supercapacitor electrode materials in an aqueous electrolyte. Dalton Trans., 2013,42(5):1603-1611.
DOI URL PMID |
| [43] | KANNANGARA Y Y, RATHNAYAKE U A, SONG J K. Redox active multi-layered Zn-pPDA MOFs as high-performance supercapacitor electrode material. Electrochim. Acta, 2019,297:145-154. |
| [44] |
CHOI K M, JEONG H M, PARK J H, et al. Supercapacitors of nanocrystalline metal-organic frameworks. ACS Nano, 2014,8(7):7451-7457.
DOI URL PMID |
| [45] | YANG J, ZHENG C, XIONG P, et al. Zn-doped Ni-MOF material with a high supercapacitive performance. J. Mater. Chem. A, 2014,2(44):19005-19010. |
| [46] | DÍAZ R, ORCAJO M G, BOTAS J A, et al. Co8-MOF-5 as electrode for supercapacitors. Mater. Lett., 2012,68:126-128. |
| [47] | GAO W, CHEN D, QUAN H, et al. Fabrication of hierarchical porous metal-organic framework electrode for aqueous asymmetric supercapacitor. ACS Sustain. Chem. Eng., 2017,5(5):4144-4153. |
| [48] |
TALIN A A, CENTRONE A, FORD A C, et al. Tunable electrical conductivity in metal-organic framework thin-film devices. Science, 2014,343(6166):66-69.
DOI URL PMID |
| [49] |
CHUI S S Y, LO S M F, CHARMANT J P, et al. A chemically functionalizable nanoporous material [Cu3(TMA)2(H2O)3]n. Science, 1999,283(5405):1148-1150.
DOI URL PMID |
| [50] | WANG K, WANG Z, XIN W, et al. Flexible long-chain-linker constructed Ni-based metal-organic frameworks with 1D helical channel and their pseudo-capacitor behavior studies. J. Power Sources, 2018,377:44-51. |
| [51] | SALUNKHE R R, KANETI Y V, KIM J, et al. Nanoarchitectures for metal-organic framework-derived nanoporous carbons toward supercapacitor applications. Accounts Chem. Res., 2016,49(12):2796-2806. |
| [52] |
WANG L, FENG X, REN L, et al. Flexible solid-state supercapacitor based on a metal-organic framework interwoven by electrochemically-deposited PANI. J. Am. Chem. Soc., 2015,137(15):4920-4923.
DOI URL PMID |
| [53] | YANG J, GANG C, CHEN D, et al. Bimetal-organic framework assisted polymerization of pyrrole involving air oxidant to prepare composite electrodes for portable energy storage. J. Mater. Chem. A, 2017,5(45):23744-23752. |
| [54] | WANG Z, GAO C, LIU Y, et al. Electrochemical performance and transformation of Co-MOF/reduced graphene oxide composite. Mater. Lett., 2017,193:216. |
| [55] | BENNETT T D, CHEETHAM A K. Amorphous metal-organic frameworks. Accounts Chem. Res., 2014,47(5):1555-1562. |
| [56] | YANG F, LI W, TANG B J. Facile synthesis of amorphous UiO-66 (Zr-MOF) for supercapacitor application. Joarnal of Alloys & Compounds, 2018,733:8-14. |
| [57] |
MCHUGH L N, MCPHERSON M J, MCCORMICK L J, et al. Hydrolytic stability in hemilabile metal-organic frameworks. Nat. Chem., 2018,10(11):1096-1102.
DOI URL PMID |
| [58] | LAN Y, LI Z, YU C, et al. Application of zeolitic imidazolate framework in supercapacitor. New Chem. Mater., 2017,45(8):8-10. |
| [59] |
LI Z, WANG W, CAO H, et al. Boron doped ZIF-67@graphene derived carbon electrocatalyst for highly efficient enzyme-free hydrogen peroxide biosensor. Adv. Mater. Tech., 2017,2(12):1700224.
DOI URL |
| [60] | LI Z, JIANG Y, WANG Z, et al. Nitrogen-rich core-shell structured particles consisting of carbonized zeolitic imidazolate frameworks and reduced graphene oxide for amperometric determination of hydrogen peroxide. Microchim. Acta, 2018,185(11):501. |
| [61] | LI Z, LAN Y, CAO H, et al. Carbon materials derived from chitosan/ cellulose cryogel-supported zeolite imidazole frameworks for potential supercapacitor application. Carbohyd. Polym., 2017,175(1):223-230. |
| [62] | LI Z, HE H, CAO H, et al. Atomic Co/Ni dual sites and Co/Ni alloy nanoparticles in N-doped porous Janus-like carbon frameworks for bifunctional oxygen electrocatalysis. Appl. Catal. B: Environ., 2019,240:112-121. |
| [63] |
GAILLAC R, PULLUMBI P, BEYER K A, et al. Liquid metal-organic frameworks. Nat. Mater., 2017,16(11):1149-1154.
DOI URL PMID |
| [1] | DING Ling, JIANG Rui, TANG Zilong, YANG Yunqiong. MXene: Nanoengineering and Application as Electrode Materials for Supercapacitors [J]. Journal of Inorganic Materials, 2023, 38(6): 619-633. |
| [2] | YANG Zhuo, LU Yong, ZHAO Qing, CHEN Jun. X-ray Diffraction Rietveld Refinement and Its Application in Cathode Materials for Lithium-ion Batteries [J]. Journal of Inorganic Materials, 2023, 38(6): 589-605. |
| [3] | CHEN Qiang, BAI Shuxin, YE Yicong. Highly Thermal Conductive Silicon Carbide Ceramics Matrix Composites for Thermal Management: a Review [J]. Journal of Inorganic Materials, 2023, 38(6): 634-646. |
| [4] | LIN Junliang, WANG Zhanjie. Research Progress on Ferroelectric Superlattices [J]. Journal of Inorganic Materials, 2023, 38(6): 606-618. |
| [5] | ZHANG Shuo, FU Qiangang, ZHANG Pei, FEI Jie, LI Wei. Influence of High Temperature Treatment of C/C Porous Preform on Friction and Wear Behavior of C/C-SiC Composites [J]. Journal of Inorganic Materials, 2023, 38(5): 561-568. |
| [6] | NIU Jiaxue, SUN Si, LIU Pengfei, ZHANG Xiaodong, MU Xiaoyu. Copper-based Nanozymes: Properties and Applications in Biomedicine [J]. Journal of Inorganic Materials, 2023, 38(5): 489-502. |
| [7] | YUAN Jingkun, XIONG Shufeng, CHEN Zhangwei. Research Trends and Challenges of Additive Manufacturing of Polymer-derived Ceramics [J]. Journal of Inorganic Materials, 2023, 38(5): 477-488. |
| [8] | DU Jianyu, GE Chen. Recent Progress in Optoelectronic Artificial Synapse Devices [J]. Journal of Inorganic Materials, 2023, 38(4): 378-386. |
| [9] | YANG Yang, CUI Hangyuan, ZHU Ying, WAN Changjin, WAN Qing. Research Progress of Flexible Neuromorphic Transistors [J]. Journal of Inorganic Materials, 2023, 38(4): 367-377. |
| [10] | YOU Junqi, LI Ce, YANG Dongliang, SUN Linfeng. Double Dielectric Layer Metal-oxide Memristor: Design and Applications [J]. Journal of Inorganic Materials, 2023, 38(4): 387-398. |
| [11] | CHEN Kunfeng, HU Qianyu, LIU Feng, XUE Dongfeng. Multi-scale Crystallization Materials: Advances in in-situ Characterization Techniques and Computational Simulations [J]. Journal of Inorganic Materials, 2023, 38(3): 256-269. |
| [12] | ZHANG Chaoyi, TANG Huili, LI Xianke, WANG Qingguo, LUO Ping, WU Feng, ZHANG Chenbo, XUE Yanyan, XU Jun, HAN Jianfeng, LU Zhanwen. Research Progress of ScAlMgO4 Crystal: a Novel GaN and ZnO Substrate [J]. Journal of Inorganic Materials, 2023, 38(3): 228-242. |
| [13] | QI Zhanguo, LIU Lei, WANG Shouzhi, WANG Guogong, YU Jiaoxian, WANG Zhongxin, DUAN Xiulan, XU Xiangang, ZHANG Lei. Progress in GaN Single Crystals: HVPE Growth and Doping [J]. Journal of Inorganic Materials, 2023, 38(3): 243-255. |
| [14] | LIN Siqi, LI Airan, FU Chenguang, LI Rongbing, JIN Min. Crystal Growth and Thermoelectric Properties of Zintl Phase Mg3X2 (X=Sb, Bi) Based Materials: a Review [J]. Journal of Inorganic Materials, 2023, 38(3): 270-279. |
| [15] | LIU Yan, ZHANG Keying, LI Tianyu, ZHOU Bo, LIU Xuejian, HUANG Zhengren. Electric-field Assisted Joining Technology for the Ceramics Materials: Current Status and Development Trend [J]. Journal of Inorganic Materials, 2023, 38(2): 113-124. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||