
Journal of Inorganic Materials ›› 2020, Vol. 35 ›› Issue (3): 345-351.DOI: 10.15541/jim20190351
Special Issue: 环境与催化材料论文精选
Previous Articles Next Articles
LIU Rong,ZHANG Wei,CHEN Yuantao( ),FAN Yuanrui,HU Guangzhang,XU Cheng,HAN Zheng
),FAN Yuanrui,HU Guangzhang,XU Cheng,HAN Zheng
Received:2019-07-15
Revised:2019-09-19
Published:2020-03-20
Online:2019-10-23
About author:LIU Rong(1995-), female, Master candidate. E-mail: 18910742916@163.com
Supported by:CLC Number:
LIU Rong, ZHANG Wei, CHEN Yuantao, FAN Yuanrui, HU Guangzhang, XU Cheng, HAN Zheng. Adsorption of Iodine by ZIF Materials[J]. Journal of Inorganic Materials, 2020, 35(3): 345-351.
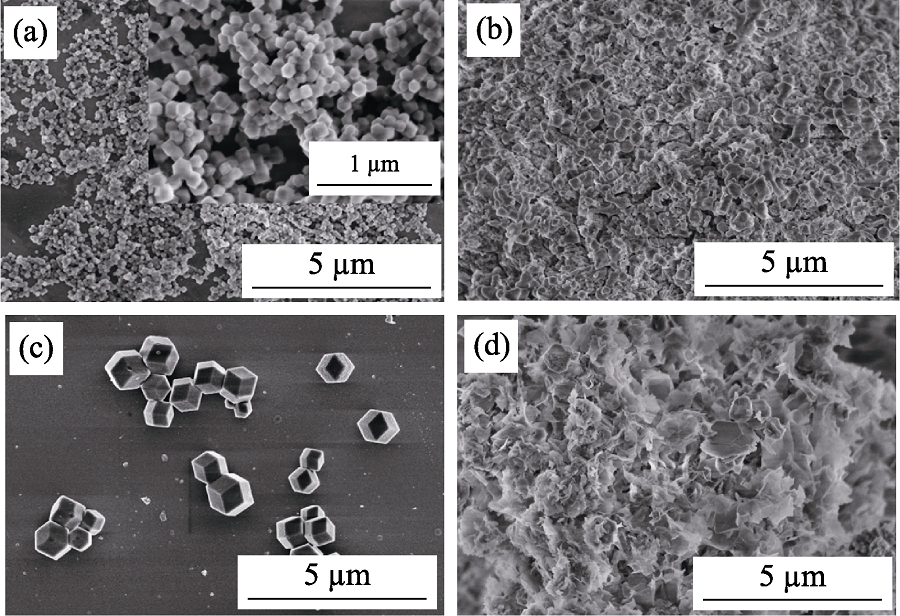
Fig. 3 SEM images of ZIF-8 and ZIF-67 before and after iodine adsorption (a) ZIF-8 before adsorption, (b) ZIF-8 after adsorption, (c) ZIF-67 before adsorption, (d) ZIF-67 after adsorption
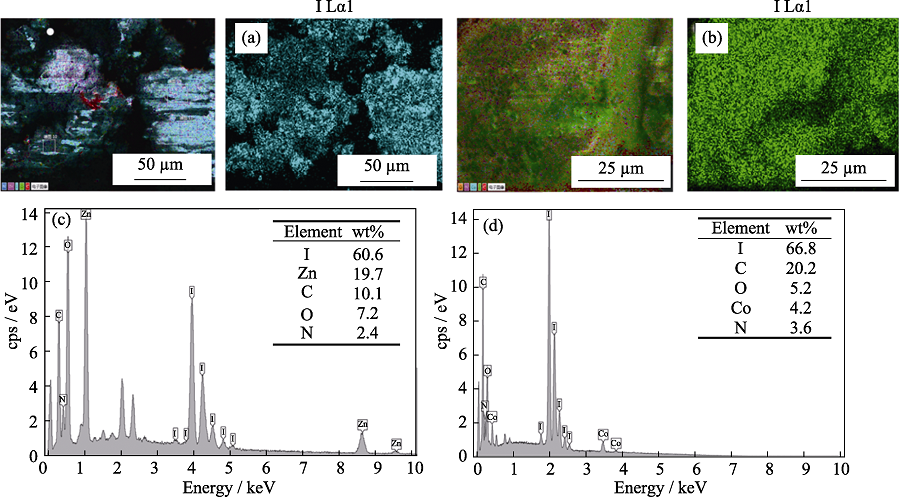
Fig. 4 Mapping images and EDS patterns of ZIF-8 and ZIF-67 (a) Mapping image of ZIF-8 after adsorption iodine; (b) Mapping image of ZIF-67 after adsorption iodine; (c) EDS pattern of ZIF-8 after adsorption iodine; (d) EDS pattern of ZIF-67 after adsorption iodine
| ZIF-8 | ZIF-67 | ||
|---|---|---|---|
| Langmuir equation | Qm/(mg·g-1) | 64416.71 | 77747.64 |
| kL/(L·mg-1) | 1.59×10-4 | 1.30×10-4 | |
| R2 | 0.99 | 0.99 | |
| Freundlich equation | kF/(mg·g-1) | 10.00 | 10.84 |
| n | 1.00 | 0.98 | |
| R2 | 1.00 | 0.99 |
Table 1 Langmuir and Freundlich parameters for adsorption of iodine by ZIF-8 and ZIF-67
| ZIF-8 | ZIF-67 | ||
|---|---|---|---|
| Langmuir equation | Qm/(mg·g-1) | 64416.71 | 77747.64 |
| kL/(L·mg-1) | 1.59×10-4 | 1.30×10-4 | |
| R2 | 0.99 | 0.99 | |
| Freundlich equation | kF/(mg·g-1) | 10.00 | 10.84 |
| n | 1.00 | 0.98 | |
| R2 | 1.00 | 0.99 |
| ZIF-8 | ZIF-67 | ||
|---|---|---|---|
| Psedo-first-order kinetics equation | Qe/(mg·g-1) | 1010.54 | 1047.37 |
| k1/(L·mg-1) | 1.30×10-4 | 1.49×10-4 | |
| R2 | 0.97 | 0.93 | |
| Psedo-second-order kinetics equation | Qe/(mg·g-1) | 947.61 | 916.08 |
| k2/(g·min-1·mg-1) | 0.08 | 0.12 | |
| R2 | 0.99 | 0.96 |
Table 2 Pseudo first and pseudo second order kinetics parameters for the adsorption of iodine by ZIF-8 and ZIF-67
| ZIF-8 | ZIF-67 | ||
|---|---|---|---|
| Psedo-first-order kinetics equation | Qe/(mg·g-1) | 1010.54 | 1047.37 |
| k1/(L·mg-1) | 1.30×10-4 | 1.49×10-4 | |
| R2 | 0.97 | 0.93 | |
| Psedo-second-order kinetics equation | Qe/(mg·g-1) | 947.61 | 916.08 |
| k2/(g·min-1·mg-1) | 0.08 | 0.12 | |
| R2 | 0.99 | 0.96 |
| [1] | VERBRUGGEN A, LAES E, LEMMENS S . Assessment of the actual sustainability of nuclear fission power. Renewable and Sustainable Energy Reviews, 2014,32:16-28. |
| [2] | MICHAEL S, VIVIANNE H M VISSCHERS . Acceptance of nuclear power: the Fukushima effect. Energy Policy, 2013,59:112-119. |
| [3] | SEKIZAWA J . Risk communication about radionuclide contamination of food after the Fukushima nuclear power plant accident. Shokuhinseigaku Zasshi Journal of the Food Hygienic Society of Japan, 2013,54(2):89. |
| [4] | KITADAA S, OIKAWAB T, WATANABEB S , et al. Removal of radioactive iodine and cesium in water purification. Desalination & Water Treatment, 2014,27(12):1-8. |
| [5] | GROUP W A . Midterm report on removal of radioactive iodine and cesium from rainwater contaminated by Fukushima daiichi nuclear accident. Radiation Safety Management, 2013,12:22-30. |
| [6] | JANG E S, KIM J S . Radionuclides in environmental samples and sample concentration of land in the analysis in the method of direct. Journal of Environmental Science International, 2015,24(3):275-280. |
| [7] | ZHDANKIN V V, STANG P J . Recent developments in the chemistry of polyvalent iodine compounds. Chemical Reviews, 2002,102(7):2523-2584. |
| [8] | BAKER A . Inorganic iodine speciation in tropical Atlantic aerosol. Geophysical Research Letters, 2004,31(23):187-206. |
| [9] | JIAHENG H, WENBIN Z, LIN J , et al. Adsorption of iodine on silver wire. Journal of Nuclear & Radiochemistry, 2010,32(2):121-125. |
| [10] | BANERJEE D, CHEN X, LOBANOV S , et al. Iodine adsorption in metal organic frameworks in the presence of humidity. ACS Applied Materials & Interfaces, 2018,10(13):10622-10626. |
| [11] | YU F, CHEN Y, WANG Y , et al. Enhanced removal of iodide from aqueous solution by ozonation and subsequent adsorption on Ag-Ag2O modified on carbon spheres. Applied Surface Science,, 2018,427:753-762. |
| [12] | PEI C, BEN T, XU S , et al. Ultrahigh iodine adsorption in porous organic frameworks. Journal of Materials Chemistry A, 2014,2(20):7179-7187. |
| [13] | SIGEN A, ZHANG Y, LI Z , et al. Highly efficient and reversible iodine capture using a metalloporphyrin-based conjugated microporous polymer. Chemical Communications, 2014,50(62):8495-8498. |
| [14] | LI H, DING X, HAN B H . Porous azo-bridged porphyrin- phthalocyanine network with high iodine capture capability. Chemistry-A European Journal, 2016,22(33):11863-11868. |
| [15] | JANETA M, BURY W, SZAFERT S . Porous silsesquioxane-imine frameworks as highly efficient adsorbents for cooperative iodine capture. ACS Applied Materials & Interfaces, 2018,10(23):19964-19973. |
| [16] | WANG Z, HUANG Y, YANG J , et al. The water-based synthesis of chemically stable Zr-based MOFs using pyridine-containing ligands and their exceptionally high adsorption capacity for iodine. Dalton Transactions, 2017,46(23):7412-7420. |
| [17] | HU Y, KAZEMIAN H, ROHANI S , et al. In situ high pressure study of ZIF-8 by FTIR spectroscopy. Chemical Communications, 2011,47(47):12694-12696. |
| [18] | LI R, CHE R, LIU Q , et al. Hierarchically structured layered-double- hydroxides derived by ZIF-67 for uranium recovery from simulated seawater. Journal of Hazardous Materials, 2017,338:167-176. |
| [19] | HAN R, ZOU W, WANG Y , et al. Removal of uranium (VI) from aqueous solutions by manganese oxide coated zeolite: discussion of adsorption isotherms and pH effect. Journal of Environmental Radioactivity, 2007,93(3):127-143. |
| [20] | FOO K Y, HAMEED B H . Insights into the modeling of adsorption isotherm systems. Chemical Engineering Journal, 2010,156(1):2-10. |
| [21] | XIE S, YANG J, CHEN C , et al. Study on biosorption kinetics and thermodynamics of uranium by Citrobacter freudii. Journal of Environmental Radioactivity, 2008,99(1):126-133. |
| [22] | MENG H, LI Z, MA F , et al. Preparation and characterization of surface imprinted polymer for selective sorption of uranium (VI). Journal of Radioanalytical and Nuclear Chemistry, 2015,306(1):139-146. |
| [23] | YANG J, ZHANG F, LU H , et al. Hollow Zn/Co ZIF particles derived from core-shell ZIF-67@ZIF-8 as selective catalyst for the semi-hydrogenation of acetylene. Angewandte Chemie International Edition, 2015,54(37):10889-10893. |
| [24] | LI Y, ZHOU K, HE M , et al. Synthesis of ZIF-8 and ZIF-67 using mixed-base and their dye adsorption. Microporous and Mesoporous Materials, 2016,234:287-292. |
| [25] | KELTY M L, MORRIS W, GALLAGHER A T , et al. High-throughput synthesis and characterization of nanocrystalline porphyrinic zirconium metal-organic frameworks. Chemical Communications, 2016,52(50):7854-7857. |
| [26] | GUPTA V K, JAIN R, MALATHI S , et al. Adsorption-desorption studies of indigocarmine from industrial effluents by using deoiled mustard and its comparison with charcoal. Journal of Colloid&Interface Science, 2010,348(2):628-633. |
| [27] | HAMEED B H, TAN I A W, AHMAD A L . Adsorption isotherm, kinetic modeling and mechanism of 2,4,6-trichlorophenol on coconut husk-based activated carbon. Chemical Engineering Journal, 2008,144(1):235-244. |
| [1] | MA Xiaosen, ZHANG Lichen, LIU Yanchao, WANG Quanhua, ZHENG Jiajun, LI Ruifeng. 13X@SiO2: Synthesis and Toluene Adsorption [J]. Journal of Inorganic Materials, 2023, 38(5): 537-543. |
| [2] | GUO Chunxia, CHEN Weidong, YAN Shufang, ZHAO Xueping, YANG Ao, MA Wen. Adsorption of Arsenate in Water by Zirconia-halloysite Nanotube Material [J]. Journal of Inorganic Materials, 2023, 38(5): 529-536. |
| [3] | WANG Shiyi, FENG Aihu, LI Xiaoyan, YU Yun. Pb (II) Adsorption Process of Fe3O4 Supported Ti3C2Tx [J]. Journal of Inorganic Materials, 2023, 38(5): 521-528. |
| [4] | YU Yefan, XU Ling, NI Zhongbing, SHI Dongjian, CHEN Mingqing. Prussian Blue Modified Biochar: Preparation and Adsorption of Ammonia Nitrogen from Sewage [J]. Journal of Inorganic Materials, 2023, 38(2): 205-212. |
| [5] | WANG Hongning, HUANG Li, QING Jiang, MA Tengzhou, HUANG Weiqiu, CHEN Ruoyu. Mesoporous Organic-inorganic Hybrid Siliceous Hollow Spheres: Synthesis and VOCs Adsorption [J]. Journal of Inorganic Materials, 2022, 37(9): 991-1000. |
| [6] | MA Lei, HUANG Yi, DENG Hao, YIN Hang, TIAN Qiang, YAN Minghao. Removal of Uranium (VI) from Acidic Aqueous Solution by Fluorapatite [J]. Journal of Inorganic Materials, 2022, 37(4): 395-403. |
| [7] | LIU Cheng, ZHAO Qian, MOU Zhiwei, LEI Jiehong, DUAN Tao. Adsorption Properties of Novel Bismuth-based SiOCNF Composite Membrane for Radioactive Gaseous Iodine [J]. Journal of Inorganic Materials, 2022, 37(10): 1043-1050. |
| [8] | ZHOU Fan, BI Hui, HUANG Fuqiang. Ultra-large Specific Surface Area Activated Carbon Synthesized from Rice Husk with High Adsorption Capacity for Methylene Blue [J]. Journal of Inorganic Materials, 2021, 36(8): 893-903. |
| [9] | YU Xiangkun, LIU Kun, LI Zhipeng, ZHAO Yulu, SHEN Jinyou, MAO Ping, SUN Aiwu, JIANG Jinlong. Efficient Adsorption of Radioactive Iodide by Copper/Palygorskite Composite [J]. Journal of Inorganic Materials, 2021, 36(8): 856-864. |
| [10] | SU Li, YANG Jianping, LAN Yue, WANG Lianjun, JIANG Wan. Interface Design of Iron Nanoparticles for Environmental Remediation [J]. Journal of Inorganic Materials, 2021, 36(6): 561-569. |
| [11] | XI Wen, LI Haibo. Preparation of TiO2/Ti3C2Tx Composite for Hybrid Capacitive Deionization [J]. Journal of Inorganic Materials, 2021, 36(3): 283-291. |
| [12] | WANG Tingting, SHI Shumei, LIU Chenyuan, ZHU Wancheng, ZHANG Heng. Synthesis of Hierarchical Porous Nickel Phyllosilicate Microspheres as Efficient Adsorbents for Removal of Basic Fuchsin [J]. Journal of Inorganic Materials, 2021, 36(12): 1330-1336. |
| [13] | GUO Yu, JIANG Xiaoqing, WU Hongmei, XIAO Yu, WU Dafu, LIU Xin. Preparation of 2-hydroxy-1-naphthalene Functionalized SBA-15 Adsorbent for the Adsorption of Chromium(III) Ions from Aqueous Solution [J]. Journal of Inorganic Materials, 2021, 36(11): 1163-1170. |
| [14] | ZHANG Ruihong, WEI Xin, LU Zhanhui, AI Yuejie. Training Model for Predicting Adsorption Energy of Metal Ions Based on Machine Learning [J]. Journal of Inorganic Materials, 2021, 36(11): 1178-1184. |
| [15] | HE Junlong, SONG Erhong, WANG Lianjun, JIANG Wan. DFT Calculation of NO Adsorption on Cr Doped Graphene [J]. Journal of Inorganic Materials, 2021, 36(10): 1047-1052. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||