
Journal of Inorganic Materials ›› 2019, Vol. 34 ›› Issue (4): 358-364.DOI: 10.15541/jim20180279
Previous Articles Next Articles
Xiao-Jing FENG1,Gong-Kai WANG1,2,3,Xiao-Ran WANG1,Jun HE1,Xin WANG1,Hui-Fen PENG1,2,3( )
)
Received:2018-06-22
Revised:2018-10-07
Published:2019-04-20
Online:2019-04-15
Supported by:CLC Number:
Xiao-Jing FENG, Gong-Kai WANG, Xiao-Ran WANG, Jun HE, Xin WANG, Hui-Fen PENG. Electrochemical Property of Cr 3+ Doped LiSn2(PO4)3 Anode Material[J]. Journal of Inorganic Materials, 2019, 34(4): 358-364.
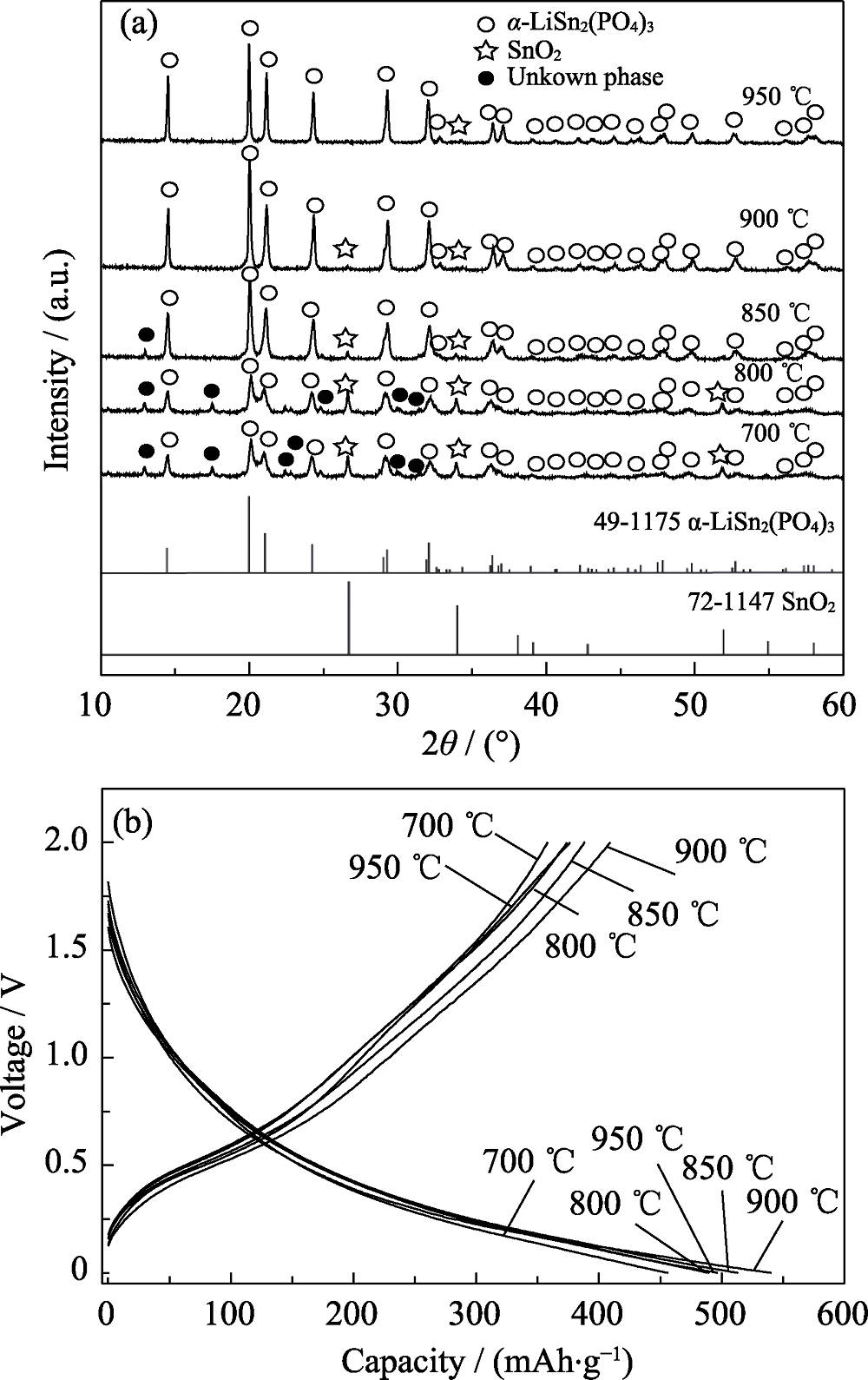
Fig. 1 XRD patterns (a) and the second charge-discharge curves at 100 mA/g (b) of the products heat-treated at various temperatures with Cr3+ content of 0.4
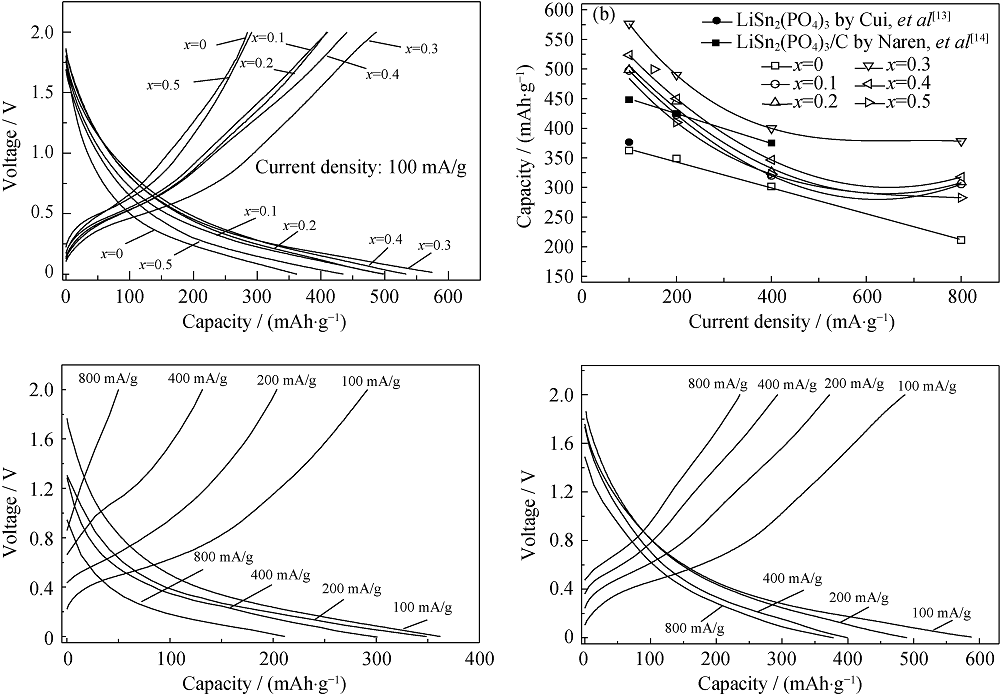
Fig. 3 Second charge-discharge curves at 100 mA/g (a) and the discharge capacities at different current densities (b) of the batteries assembled with the samples containing different Cr3+ contents, and the charge-discharge curves at different rates for the samples of Li1Sn2(PO4)3 (c) and Li1.3Cr0.3Sn1.7(PO4)3 (d)
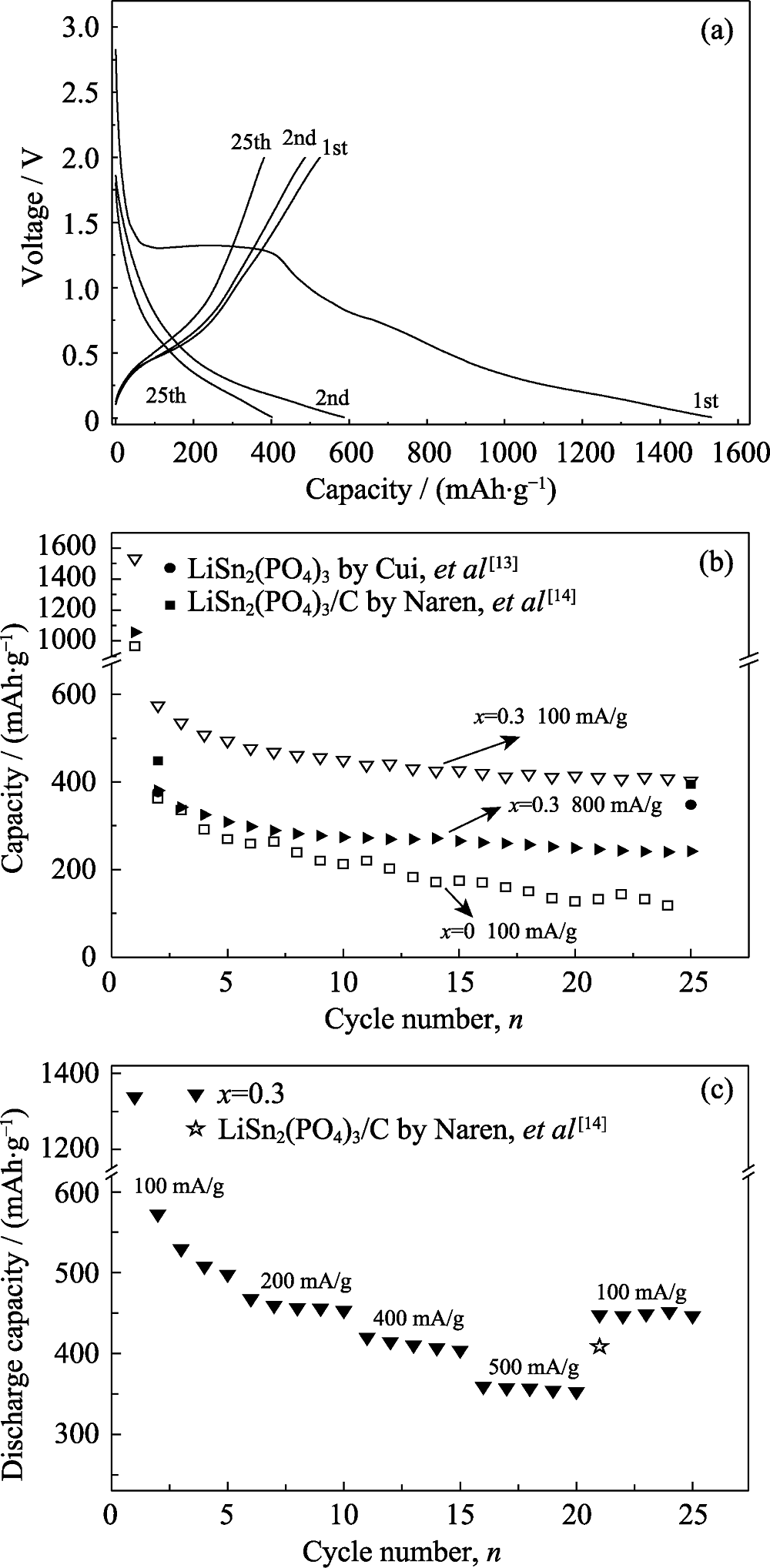
Fig. 4 Cyclic charge-discharge curves at 100 mA/g of the batteries assembled with the sample Li1.3Cr0.3Sn1.7(PO4)3 (a), variation in discharging capacity for the samples of x=0 and x=0.3 (b) and rate performance between 100 mA/g and 800 mA/g for the batteries assembled with the Li1.3Cr0.3Sn1.7(PO4)3 sample (c)
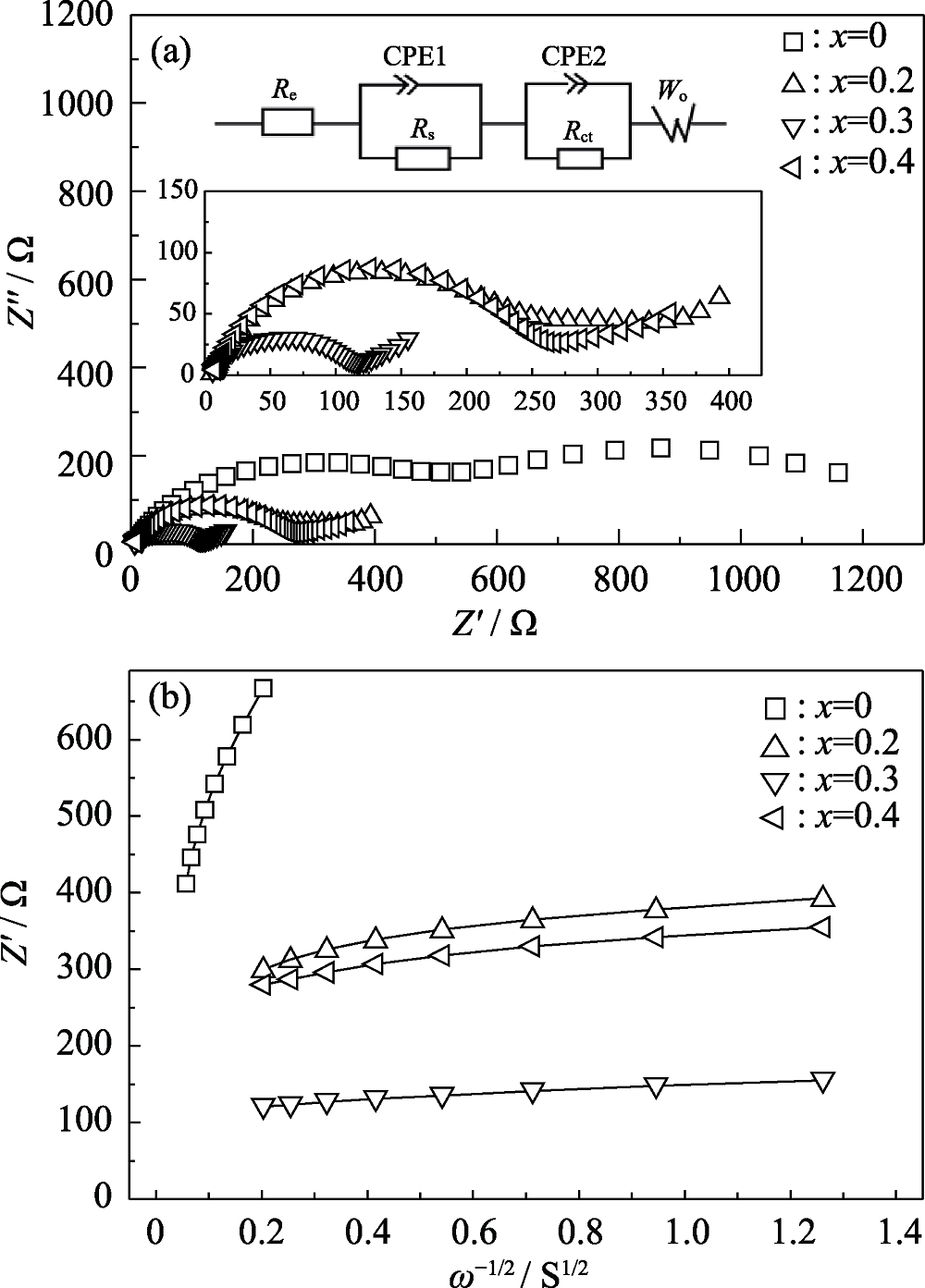
Fig. 5 AC impedance spectra (a) and relationship between Z° and ω-1/2of the samples with different Cr3+ contents (b) with inserts showing local magnification of AC impedance spectra and their equivalent circuit
| Cr3+content | Rct/Ω | σ/(Ω·cm2·s-1/2) | D/(cm2·s-1) |
|---|---|---|---|
| x=0 | 563.3 | 1698.0 | 2.0×10-14 |
| x=0.2 | 260.0 | 83.4 | 8.3×10-12 |
| x=0.3 | 114.7 | 32.5 | 5.5×10-11 |
| x=0.4 | 252.2 | 70.8 | 1.6×10-11 |
Table 1 Charge transfer resistance (Rct), Warburg coefficients (σ) in Fig. 5 and Li+ diffusion coefficients (D) of the samples
| Cr3+content | Rct/Ω | σ/(Ω·cm2·s-1/2) | D/(cm2·s-1) |
|---|---|---|---|
| x=0 | 563.3 | 1698.0 | 2.0×10-14 |
| x=0.2 | 260.0 | 83.4 | 8.3×10-12 |
| x=0.3 | 114.7 | 32.5 | 5.5×10-11 |
| x=0.4 | 252.2 | 70.8 | 1.6×10-11 |
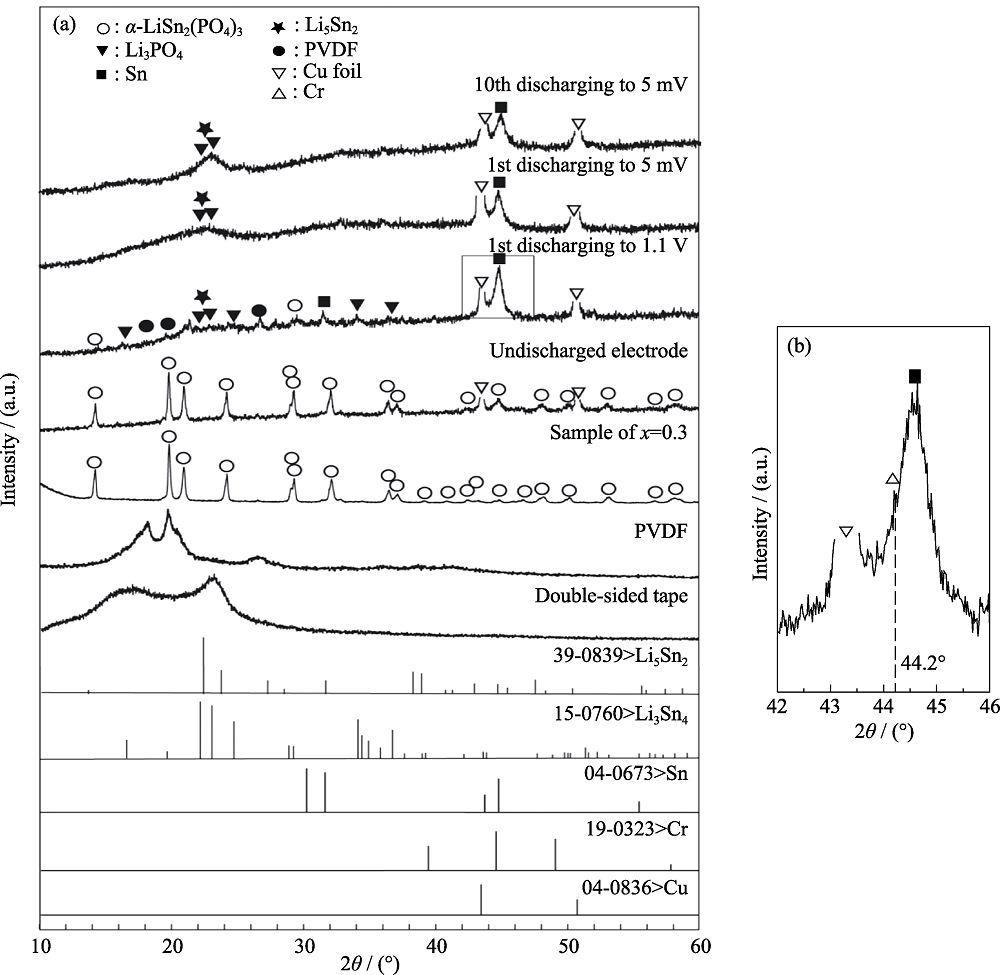
Fig.S3 XRD patterns of the Li1.3Cr0.3Sn1.7(PO4)3 electrode after charging and discharging at different voltages (a), and enlargement (b) of the box region in (a)
| [1] | LAI Y Q, DU S L, AI L H , et al. Insight into heat generation of lithium ion batteries based on the electrochemical-thermal model at high discharge rates. Int.[J]. Hydrogen. Energ., 2015,40(38):13039-13049. |
| [2] | ROY P, SRIVASTAVA S K . Nanostructured anode materials for lithium ion batteries. J. Mater. Chem.A, 2015,3(6):2454-2484. |
| [3] | BEHM M, IRVINE T S . Influence of structure and composition upon performance of tin phosphate based negative electrodes for lithium batteries. Electrochim.Acta, 2002,47(11):1727-1738. |
| [4] | NITHYADHARSENI P, REDDY M V, NALINI B , et al. Sn-based intermetallic alloy anode materials for the application of lithium ion batteries. Electrochim.Acta, 2015,161(1):261-268. |
| [5] | SHARMA Y, SHARMA N, RAO G V S , et al. Studies on nano-CaO·SnO2 and nano-CaSnO3 as anodes for Li-ion batteries. Chem.Mater. 2016,20(21):6829-6839. |
| [6] | UI K, KIKUCHI S, KADOMA Y , et al. Electrochemical characteristics of Sn film prepared by pulse electrodeposition method as negative electrode for lithium secondary batteries.[J]. Power Sources, 2009,189(1):224-229. |
| [7] | TRAHEY L, VAUGHEY J T, KUNG H H , et al. High-capacity microporous Cu6Sn5-Sn anodes for Li-ion batteries.[J]. Electrochem. Soc., 2009,156(5):A385-A389. |
| [8] | JAVADIAN S, KAKEMAM J, GHARIBI H , et al. Flower-like architecture of CoSn4 nano structure as anode in lithium ion batteries. Int.[J]. Hydrogen Energ., 2017,42:13126-13149. |
| [9] | SENGUPTA S, PATRA A, AKHTAR M , et al. 3D microporous Sn-Sb-Ni alloy impregnated Ni foam as high-performance negative electrode for lithium-ion batteries.[J]. Alloys Compd., 2017,705:290-300. |
| [10] | ZHANG H, ZHANG M R, ZHANG M L , et al. Hybrid aerogel-derived Sn-Ni alloy immobilized within porous carbon/ graphene dual matrices for high-performance lithium storage.[J]. Colloid Interf. Sci., 2017,501:267-272. |
| [11] | YE Y, WU P, ZHANG X , et al. Facile synthesis of graphene supported FeSn2 nanocrystals with enhanced Li-storage capability. RSC Advances, 2014,4(33):17401-17404. |
| [12] | HUGGINS R A . Lithium alloy negative electrodes. J. Power Sources, 1999, 81-82(1/2):13-19. |
| [13] | CUI W J, YI J, CHEN L , et al. Synjournal and electrochemical characteristics of NASICON-structured LiSn2(PO4)3 anode material for lithium-ion batteries.[J]. Power Sources, 2012,217(11):77-84. |
| [14] | NAREN, TIAN J H, WANG D D , et al. Improved electrochemical performances of LiSn2(PO4)3 anode material for lithium-ion battery prepared by solid-state method.[J]. Power Sources, 2017,361(1):96-104. |
| [15] | NORHANIZA R, SUBBAN R H Y, MOHAMED N S , et al. Chromium substituted LiSn2P3O12 solid electrolyte. Int.[J]. Electrochem. Sc., 2012,7(10):10254-10265. |
| [16] | ZHANG P, WANG H, SI Q , et al. High lithium ion conductivity solid electrolyte of chromium and aluminum co-doped NASICON-type LiTi2(PO4)3. Solid State Ionics, 2015,272:101-106. |
| [17] | ARGON M J, LAVELA P, ORTIZG F , et al. Benefits of chromium substitution in Na3V2(PO4)3 as a potential candidate for sodium-ion batteries. ChemElectroChem, 2015,2(7):995-1002. |
| [18] | PLYLAHAN N, VIDAL-ABARCA C, LAVELA P , et al. Chromium substitution in ion exchanged Li3Fe2(PO4)3 and the effects on the electrochemical behavior as cathodes for lithium batteries. Electrochim. Acta, 2012,62(1):124-131. |
| [19] | PRATTA R, BLOWES D W, PTACEK C . Products of chromate reduction on proposed subsurface remediation material.[J]. Environ. Sci. Technol., 1997,31:2492-2498. |
| [20] | ZHANG B, HE J, HUA Z S , et al. Effect of MoO42- doping on electrochemicalproperties of the Nasicon Li3Fe2(PO4)3 cathode. Chinese J. Inorg. Chem., 2016,32(12):2109-2116. |
| [21] | YANG Y G, ZHANG Y G, HUA Z S , et al. Effect of VO43- substitution for PO43- on electrochemical properties of the Li3Fe2(PO4)3 cathode materials. Electrochim.Acta, 2016,219(1):547-552. |
| [22] | GENG S X, YANG Y G, ZHANG Y G , et al. Effect of VO43- substitution for PO43- on electrical conductivity in the Nasicon Li3Sc2(PO4)3 compound. Electrochim.Acta, 2015,219(1):327-333. |
| [1] | WANG Yutong, ZHANG Feifan, XU Naicai, WANG Chunxia, CUI Lishan, HUANG Guoyong. Research Progress of LiTi2(PO4)3 Anode for Aqueous Lithium-ion Batteries [J]. Journal of Inorganic Materials, 2022, 37(5): 481-492. |
| [2] | WANG Jing, XU Shoudong, LU Zhonghua, ZHAO Zhuangzhuang, CHEN Liang, ZHANG Ding, GUO Chunli. Hollow-structured CoSe2/C Anode Materials: Preparation and Sodium Storage Properties for Sodium-ion Batteries [J]. Journal of Inorganic Materials, 2022, 37(12): 1344-1350. |
| [3] | LI Kunru, HU Xinghui, ZHANG Zhengfu, GUO Yuzhong, HUANG Ruian. Three-dimensional Porous Biogenic Si/C Composite for High Performance Lithium-ion Battery Anode Derived from Equisetum Fluviatile [J]. Journal of Inorganic Materials, 2021, 36(9): 929-935. |
| [4] | ZHAN Jing,XU Changfan,LONG Yiyu,LI Qihou. Bi2Mn4O10: Preparation by Polyacrylamide Gel Method and Electrochemical Performance [J]. Journal of Inorganic Materials, 2020, 35(7): 827-833. |
| [5] | XIA Tian, MENG Xie, LUO Ting, ZHAN Zhongliang. La 3+-substituted Sr2Fe1.5Ni0.1Mo0.4O6-δ as Anodes for Solid Oxide Fuel Cells [J]. Journal of Inorganic Materials, 2020, 35(5): 617-622. |
| [6] | ZHU Zeyang,WEI Jishi,HUANG Jianhang,DONG Xiangyang,ZHANG Peng,XIONG Huanming. Preparation of ZnO Nanorods with Lattice Vacancies and Their Application in Ni-Zn Battery [J]. Journal of Inorganic Materials, 2020, 35(4): 423-430. |
| [7] | ZHENG Shiyou, DONG Fei, PANG Yuepeng, HAN Pan, YANG Junhe. Research Progress on Nanostructured Metal Oxides as Anode Materials for Li-ion Battery [J]. Journal of Inorganic Materials, 2020, 35(12): 1295-1306. |
| [8] | GUO Si-Lin, KANG Shuai, LU Wen-Qiang. Ge Nanoparticles in MXene Sheets: One-step Synthesis and Highly Improved Electrochemical Property in Lithium-ion Batteries [J]. Journal of Inorganic Materials, 2020, 35(1): 105-111. |
| [9] | Yi TAN, Kai WANG. Silicon-based Anode Materials Applied in High Specific Energy Lithium-ion Batteries: a Review [J]. Journal of Inorganic Materials, 2019, 34(4): 349-357. |
| [10] | HU Xi, LIU Hong-Bo, XIA Xiao-Hong, GU Zhi-Qiang. Polyaniline-carbon Pillared Graphene Composite: Preparation and Electrochemical Performance [J]. Journal of Inorganic Materials, 2019, 34(2): 145-151. |
| [11] | TAN Yi, XUE Bing. Research Progress on Lithium Titanate as Anode Material in Lithium-ion Battery [J]. Journal of Inorganic Materials, 2018, 33(5): 475-482. |
| [12] | XIAO Na, PANG Yang, SONG Yun, WU Xiao-Jing, FU Zheng-Wen, ZHOU Yong-Ning. Electrochemical Behavior of Sb-Si Nanocomposite Thin Films as Anode Materials for Sodium-ion Batteries [J]. Journal of Inorganic Materials, 2018, 33(5): 494-500. |
| [13] | WANG Hao, LUO Yong-Chun, DENG An-Qiang, ZHAO Lei, JIANG Wan-Ting. Annealing Temperature on Structural and Electrochemical Property of Mg-free La-Y-Ni Based A2B7-type Hydrogen Storage Alloys [J]. Journal of Inorganic Materials, 2018, 33(4): 434-440. |
| [14] | CAI Jian-Xin, LI Zhi-Peng, LI Wei, ZHAO Peng-Fei, YANG Zhen-Yu, YU Ji. Synthesis and Electrochemical Performance of Fe2O3 Nanofibers as Anode Materials for LIBs [J]. Journal of Inorganic Materials, 2018, 33(3): 301-306. |
| [15] | ZENG Yan-Fei, XIN Guo-Xiang, BULIN Chao-Ke, ZHANG Bang-Wen. One-step Preparation and Electrochemical Performance of 3D Reduced Graphene Oxide/NiO as Supercapacitor Electrodes Materials [J]. Journal of Inorganic Materials, 2018, 33(10): 1070-1076. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||