
Journal of Inorganic Materials ›› 2019, Vol. 34 ›› Issue (2): 193-200.DOI: 10.15541/jim20180132
• RESEARCH PAPER • Previous Articles Next Articles
SUI Li-Li1, WANG Run1, ZHAO Dan2, SHEN Shu-Chang1, SUN Li1, XU Ying-Ming2, CHENG Xiao-Li2, HUO Li-Hua2
Received:2018-03-30
Revised:2018-06-05
Published:2019-02-20
Online:2019-01-24
About author:SUI Li-Li. E-mail: sui_leelee@126.com
Supported by:CLC Number:
SUI Li-Li, WANG Run, ZHAO Dan, SHEN Shu-Chang, SUN Li, XU Ying-Ming, CHENG Xiao-Li, HUO Li-Hua. Construction of Hierarchical α-MoO3 Hollow Microspheres and Its High Adsorption Performance towards Organic Dyes[J]. Journal of Inorganic Materials, 2019, 34(2): 193-200.
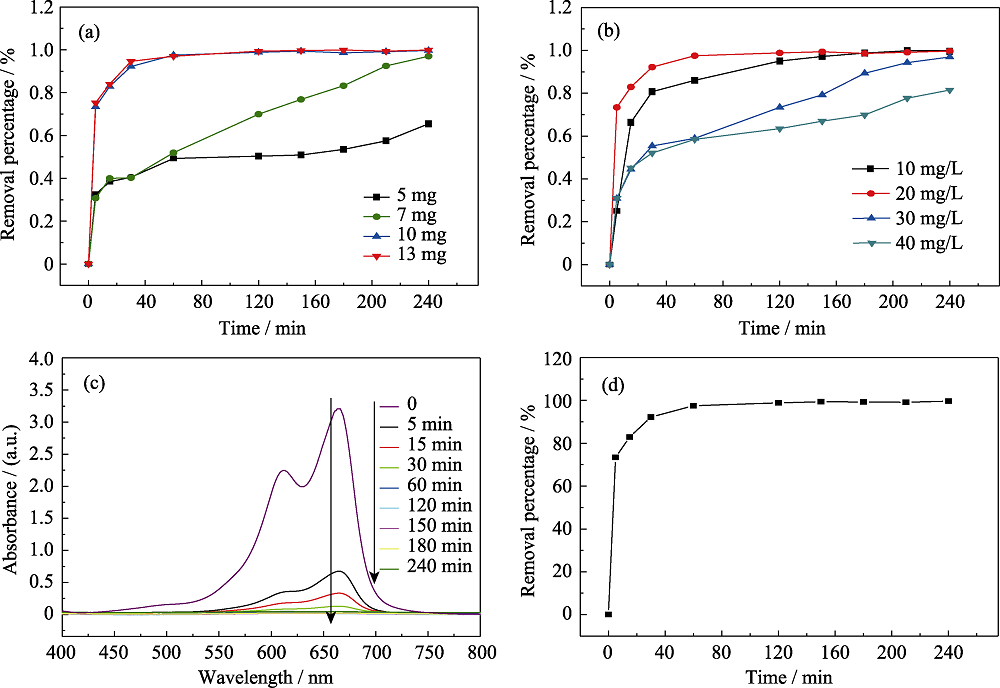
Fig. 6 Effect of adsorbent dosage (a) and concentration of MB (b) on the adsorption performances of α-MoO3 hollow microspheres, UV-Vis spectra (c) and removal percentage curves (d) of α-MoO3 to MB for different contact times
| C0/(mg·L-1) | qe.exp/(mg·g-1) | Pseudo-second-order | Pseudo-first-order | |||||
|---|---|---|---|---|---|---|---|---|
| qe.cal/(mg·g-1) | k2/(×10-3, g·mg-1·min-1) | R2 | qe.cal/(mg·g-1) | k1 /(×10-3, min-1) | R2 | |||
| 10 | 19.97 | 20.83 | 4.10 | 0.9996 | 6.98 | 10.13 | 0.6385 | |
| 20 | 39.86 | 40.16 | 10.90 | 0.9999 | 5.34 | 20.45 | 0.8994 | |
| 30 | 58.16 | 59.85 | 0.63 | 0.9907 | 44.67 | 13.04 | 0.9456 | |
| 40 | 65.22 | 65.10 | 0.82 | 0.9903 | 36.89 | 9.30 | 0.9308 | |
Table 1 Kinetic parameters for adsorption of MB on the samples of α-MoO3 hollow microspheres
| C0/(mg·L-1) | qe.exp/(mg·g-1) | Pseudo-second-order | Pseudo-first-order | |||||
|---|---|---|---|---|---|---|---|---|
| qe.cal/(mg·g-1) | k2/(×10-3, g·mg-1·min-1) | R2 | qe.cal/(mg·g-1) | k1 /(×10-3, min-1) | R2 | |||
| 10 | 19.97 | 20.83 | 4.10 | 0.9996 | 6.98 | 10.13 | 0.6385 | |
| 20 | 39.86 | 40.16 | 10.90 | 0.9999 | 5.34 | 20.45 | 0.8994 | |
| 30 | 58.16 | 59.85 | 0.63 | 0.9907 | 44.67 | 13.04 | 0.9456 | |
| 40 | 65.22 | 65.10 | 0.82 | 0.9903 | 36.89 | 9.30 | 0.9308 | |
| Adsorption | Langmuir model | Freundlich model | |||||
|---|---|---|---|---|---|---|---|
| qm/(mg·g-1) | KL/(L·mg-1) | RL | R2 | KF/(L·g-1) | n | R2 | |
| MB | 1543.2 | 1.19 | 0.0014 | 0.9978 | 724.44 | 1.82 | 0.9035 |
Table 2 Adsorption isotherm parameters of α-MoO3 hollow microspheres to MB
| Adsorption | Langmuir model | Freundlich model | |||||
|---|---|---|---|---|---|---|---|
| qm/(mg·g-1) | KL/(L·mg-1) | RL | R2 | KF/(L·g-1) | n | R2 | |
| MB | 1543.2 | 1.19 | 0.0014 | 0.9978 | 724.44 | 1.82 | 0.9035 |
| Adsorbent | Maximum adsorption capacity, qm/(mg·g-1) | Ref. |
|---|---|---|
| Hierarchical α-MoO3 hollow microspheres | 1543.2 | This work |
| WO3 nanotube | 75.0 | [26] |
| WO3 nanorods | 73.0 | [27] |
| WO3 hollow spheres | 138.9 | [28] |
| Hierarchical WO3 hydrates | 274.3 | [8] |
| SiO2 nanoparticles | 679.9 | [29] |
| Fe3O4@Ag/SiO2 nanospheres | 128.5 | [30] |
Table 3 Comparison of the maximum adsorption capacities for MB on adsorbents of different metal oxides
| Adsorbent | Maximum adsorption capacity, qm/(mg·g-1) | Ref. |
|---|---|---|
| Hierarchical α-MoO3 hollow microspheres | 1543.2 | This work |
| WO3 nanotube | 75.0 | [26] |
| WO3 nanorods | 73.0 | [27] |
| WO3 hollow spheres | 138.9 | [28] |
| Hierarchical WO3 hydrates | 274.3 | [8] |
| SiO2 nanoparticles | 679.9 | [29] |
| Fe3O4@Ag/SiO2 nanospheres | 128.5 | [30] |
| [1] | KUMAR K Y, ARCHANAr S, VINUTH T N,et al. Superb adsorption capacity of hydrothermally synthesized copper oxide and nickel oxide nanoflakes towards anionic and cationic dyes. J. Sci.: Adv. Mater. Devices, 2017, 2(2): 183-191. |
| [2] | JIN Y J, LI N, LIU H Q,et al. Highly efficient degradation of dye pollutants by Ce-doped MoO3 catalyst at room temperature. Dalton Trans., 2014, 43(34): 12860-12870. |
| [3] | HOKKANEN S, BHATNAGAR A, SILLANPAA M.A review on modification methods to cellulose-based adsorbents to improve adsorption capacity.Water Res., 2016, 91: 156-173. |
| [4] | TIAN P, HAN X Y, NING G L,et al. Synthesis of porous hierarchical MgO and its superb adsorption properties. ACS Appl. Mater. Interfaces, 2013, 5(23): 12411-12418. |
| [5] | RONG X S, QIU F X, QIN J,et al. A facile hydrothermal synthesis, adsorption kinetics and isotherms to Congo Red azo-dye from aqueous solution of NiO/grapheme nanosheets adsorbent. J. Indust. Eng. Chem., 2015, 26: 354-363. |
| [6] | SONG L X, YANG Z K, TENG Y,et al. Nickel oxide nanoflowers: formation, structure, magnetic property and adsorptive performance towards organic dyes and heavy metal ions. J. Mater. Chem. A, 2013, 1(31): 8731-8736. |
| [7] | ZHU D Z, ZHANG J, SONG J M,et al. Efficient one-pot synthesis of hierarchical flower-like α-Fe2O3 hollow sphereswith excellent adsorption performance for water treatment. Appl Surf. Sci., 2013, 284: 855-861. |
| [8] | LIU B X, WANG J S, WU J S,et al. Controlled fabrication of hierarchical WO3 hydrates with excellent adsorption performance. J. Mater. Chem. A, 2014, 2(6): 1947-1954. |
| [9] | LEE J H.Gas sensors using hierarchical and hollow oxide nanostructures: overview. Sens. Actuators, B, 2009, 140(1): 319-336. |
| [10] | LIU Y, FENG P Z, WANG Z,et al. Novel fabrication and enhanced photocatalytic MB degradation of hierarchical porous monoliths of MoO3 nanoplates. Sci. Rep., 2017, 7(1): 1845-1854. |
| [11] | WANG M, SONG X X, CHENG X L,et al. Highly selective and efficient adsorption dyes selfassembled by 3D hierarchical architecture of molybdenum oxide. RSC Adv., 2015, 5(104): 85248-85255. |
| [12] | SUI L L, ZHANG X F, CHENG X L,et al.Au-Loaded hierachical MoO3 hollow spheres with enhanced gas sensing performance for the detection of BTX (benzene, toluene, and xylene) and the sensing mechanism. ACS Appl. Mater. Interfaces, 2017, 9(2): 1661-1670. |
| [13] | ZHANG J, SONG P, LI J,et al. Template-assisted synthesis of hierarchical MoO3 microboxes and their high gas-sensing performance. Sens. Actuators, B, 2017, 249: 458-466. |
| [14] | XIA Y C, WU C S, ZHAO N Y,et al. hierarchical nanostructures for excellent performance ethanol sensor. Mater. Lett., 2016, 171: 117-120. |
| [15] | YAN H H, SONG P, ZHANG S,et al. Facile fabrication and enhanced gas sensing properties of hierarchical MoO3 nanostructures. RSC Adv., 2015, 5(89): 72728-72735. |
| [16] | WANG S T, ZHANG Y G, MA X C,et al. Hydrothermal route to single crystalline α-MoO3 nanobelts and hierarchical structures. Solid State Commun., 2005, 136(5): 283-287. |
| [17] | YU X Y, ZHANG G X, LU Z Y,et al. Green sacrificial template fabrication of hierarchical MoO3 nanostructures. CrystEngComm, 2014, 16(19): 3935-3939. |
| [18] | LIANG R L, CAO H Q, QIAN D,et al. MoO3 nanowires as electrochemical pseudocapacitor materials. Chem. Commun., 2011, 47(37): 10305-10307. |
| [19] | JIAN J B, LIU J L, PENG S J,et al. Facile synthesis of α-MoO3 nanobelts and their pseudocapacitive behavior in an aqueous Li2SO4 solution. J. Mater. Chem. A., 2013, 1(7): 2588-2594. |
| [20] | CHEN D L, LIU M N, YIN L,et al. Single-crystalline MoO3 nanoplates: topochemical synthesis and enhanced ethanol-sensing performance. J. Mater. Chem., 2011, 21(25): 9332-9342. |
| [21] | XU B Y, LI Y, WANG G F,et al. In situ synthesis and high adsorption performance of MoO2/Mo4O11 and MoO2/MoS2 composite nanorods by reduction of MoO3. Dalton Trans., 2015, 44(13): 6224-6228. |
| [22] | LEI C S, ZHU X F, ZHU B C,et al. Hierarchical NiO-SiO2 composite hollow microspheres with enhanced adsorption affinity towards Congo red in water. J. Colloid Inter. Sci., 2016, 466: 238-246. |
| [23] | ZHANG P P, MA X M, GUO Y M, et al. Size-controlled synthesis of hierarchical NiO hollow microspheres. Size-controlled synthesis of hierarchical NiO hollow microspheres and the adsorption for Congo red in water. Chem. Eng. J., 2012, 189-190(5): 188-195. |
| [24] | DHANAVEL S, NIVETHAA E A K, DHANAPA K,et al. α-MoO3/polyaniline composite for effective scavenging of Rhodamine B, Congo red and textile dye effluent. RSC Adv., 2016, 6(34): 28871-28886. |
| [25] | MA Y, JIA Y L, JIA Z B,et al. Facile synthesize α-MoO3 nanobelts with high adsorption property. Mater. Lett., 2015, 157: 53-56. |
| [26] | LI J, LIU X H, HAN Q F,et al. Formation of WO3 nanotube-based bundles directed by NaHSO4 and its application in water treatment. J. Mater. Chem. A, 2013, 1(7): 1246-1253. |
| [27] | ZHU J, WANG S L, XIE S H,et al. Hexagonal single crystal growth of WO3 nanorods along a [110] axis with enhanced adsorption capacity. Chem. Commun., 2011, 47(15): 4403-4405. |
| [28] | JEON S, YONG K.Morphology-controlled synthesis of highly adsorptive tungsten oxide nanostructures and their application to water treatment.J. Mater. Chem., 2010, 20(45): 10146-10151. |
| [29] | PERES E C, SLAVIERO J C, CUNHA A M,et al. Microwave synthesis of silica nanoparticles and its application for methylene blue adsorption. J. Environ. Chem. Eng., 2018, 6(1): 649-659. |
| [30] | SAINI J, GARG V K, GUPTA R K.Removal of methylene blue from aqueous solution by Fe3O4@Ag/SiO2 nanospheres: synthesis, characterization and adsorption performance. J. Mol. Liq., 2018, 250: 413-422. |
| [1] | CHI Congcong, QU Panpan, REN Chaonan, XU Xin, BAI Feifei, ZHANG Danjie. Preparation of SiO2@Ag@SiO2@TiO2 Core-shell Structure and Its Photocatalytic Degradation Property [J]. Journal of Inorganic Materials, 2022, 37(7): 750-756. |
| [2] | ZHOU Fan, BI Hui, HUANG Fuqiang. Ultra-large Specific Surface Area Activated Carbon Synthesized from Rice Husk with High Adsorption Capacity for Methylene Blue [J]. Journal of Inorganic Materials, 2021, 36(8): 893-903. |
| [3] | CHENG Fu-Qiang,JI Tian-Tian,XUE Min,MENG Zi-Hui,WU Yu-Kai. Thiohydroxy-functionalized Mesoporous Materials: Preparation and its Adsorption to Cr6+ [J]. Journal of Inorganic Materials, 2020, 35(2): 193-198. |
| [4] | ZHANG Xiao-Feng,ZHANG Guan-Hua,MENG Yue,XUE Ji-Long,XIA Sheng-Jie,NI Zhe-Ming. Photocatalytic Degradation of Methylene Blue by Schiff-base Cobalt Modified CoCr Layered Double Hydroxides [J]. Journal of Inorganic Materials, 2019, 34(9): 974-982. |
| [5] | ZHANG Yi-Qing, LIU Li, ZHANG Shu-Juan, WAN Zheng-Rui, LIU Hong-Ying, ZHOU Li-Qun. Preparation and Dehydrogenation Property of NH2-UIO-66 Supported RuCuMo Nanocatalyst [J]. Journal of Inorganic Materials, 2019, 34(12): 1316-1324. |
| [6] | ZHAO Hai-Bing, XU Hai-Feng, YANG Ke-Wei, LIN Chen-Xue, FENG Miao, YU Yan. Enhanced Photoreversible Color Switching of Methylene Blue Catalyzed by Magnesium-doped TiO2 Nanocrystals [J]. Journal of Inorganic Materials, 2018, 33(10): 1124-1130. |
| [7] | YU Yang, TONG Ming-Xing, HE Yu-Lan, CHEN Hui, GAO Jing, LI Guo-Hua. Preparation and Visible-light Photocatalytic Performance of Mesoporous Hollow TiO2/WO3 Spheres [J]. Journal of Inorganic Materials, 2017, 32(4): 365-371. |
| [8] | ZHAO Yang-Bo, SANG Li-Xia. TiO2 Nanoring/Nanotube Hierarchical Structure Growth Mechanism and Optical Absorption Property [J]. Journal of Inorganic Materials, 2017, 32(12): 1327-1331. |
| [9] | ZOU Jian-Peng, YANG Hong-Zhi, XIAO Ping, PAN Yi-Feng. Controllable Fabrication of Calcium Carbonate Hollow Microspheres with Micro-nano Hierarchical Structure [J]. Journal of Inorganic Materials, 2016, 31(7): 711-718. |
| [10] | WU Xuan-Rong, YANG Qiao-Zhen, ZHAO Yong-Xiang, LU Yan-Luo. Hydrothermal/Solvothermal Synthesis and Photocatalytic Performance of ZnS Microspheres [J]. Journal of Inorganic Materials, 2016, 31(5): 473-478. |
| [11] | BAO Xiao-Hui, MING Ping-Mei, BI Xiang-Yang. Preparation of Superhydrophobic Surface Based on SiC Particulate Reinforced Composite [J]. Journal of Inorganic Materials, 2016, 31(4): 383-387. |
| [12] | LI Bin, LI Ying-Lian, MO Shu-Yi, CHEN Ming-Guang, WANG Dong-Sheng, LONG Fei. Synthesis of Core-shell Structure In2Se3/Cuse Micro/Nano-powders Followed by Coated-rapid Heating Treatment Method for Preparing CuInSe2 Thin Films [J]. Journal of Inorganic Materials, 2016, 31(10): 1135-1140. |
| [13] | XU Ming-Li, DUAN Ben, ZHANG Ying-Jie, YANG Guo-Tao, DONG Peng, XIA Shu-Biao, YANG Xian-Wan. Effect of Modification Factors of MWCNTs Support on Electrocatalytic Performance of Pt Nanoparticles [J]. Journal of Inorganic Materials, 2015, 30(9): 931-936. |
| [14] | LU Qing, HUA Luo-Guang, CHEN Yi-Lin, GAO Bi-Fen, LIN Bi-Zhou. Preparation and Property of Oxygen-deficient Bi2WO6-x Photocatalyst Active in Visible Light [J]. Journal of Inorganic Materials, 2015, 30(4): 413-419. |
| [15] | PENG Dan, ZHENG Xue-Jun, XIE Shu-Fan, LUO Xiao-Ju, WANG Ding. Fabrication and Photocatalytic Performance of GaN/ZnO Composites [J]. Journal of Inorganic Materials, 2014, 29(9): 956-960. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||