
Journal of Inorganic Materials ›› 2018, Vol. 33 ›› Issue (10): 1035-1045.DOI: 10.15541/jim20180003
• REVIEW • Next Articles
CHU Zeng-Yong, LI Gao-Lin, JIANG Zhen-Hua, WANG Chun-Hua
Received:2018-01-02
Revised:2018-03-05
Published:2018-10-20
Online:2018-09-25
Supported by:CLC Number:
CHU Zeng-Yong, LI Gao-Lin, JIANG Zhen-Hua, WANG Chun-Hua. Recent Progress in High-quality Perovskite CH3NH3PbI3 Single Crystal[J]. Journal of Inorganic Materials, 2018, 33(10): 1035-1045.
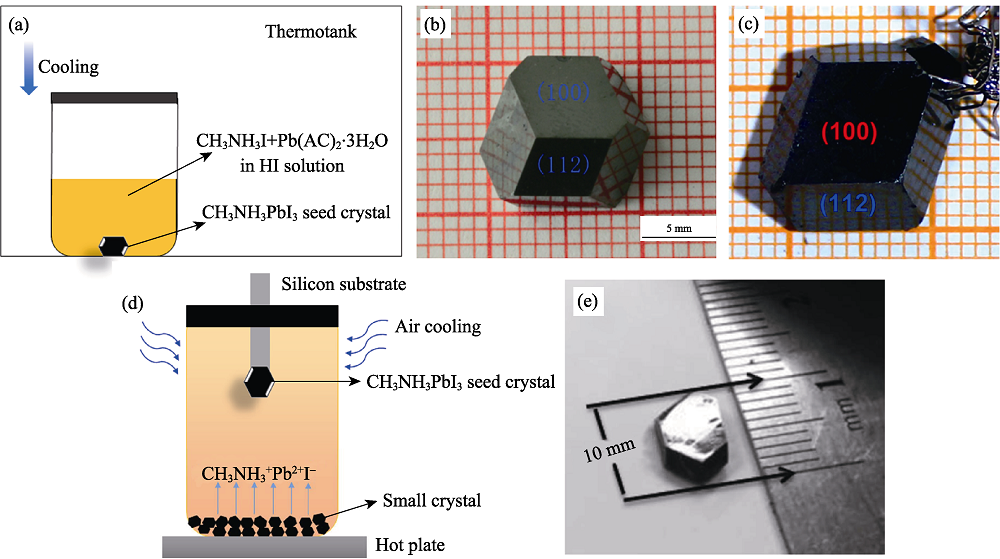
Fig. 4 Schematic diagram of solution temperature-lowering crystallization(STL) (a)-(c) Crystallization process of BSSG and images of as-prepared CH3NH3PbI3 single crystal[38, 42]; (d)-(e) Crystallization process of TSSG and image of as-prepared CH3NH3PbI3 single crystal[40]
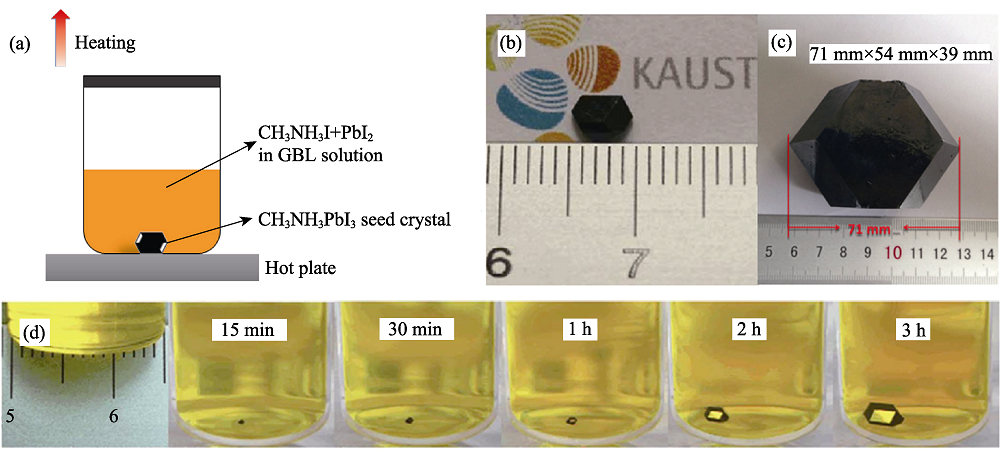
Fig. 5 Schematic diagram of inverse temperature crystallization (ITC) (a)-(c) Crystallization process of ITC and images of as-prepared CH3NH3PbI3 single crystal[41, 43]; (d) CH3NH3PbI3 crystal growth at different time intervals by ITC[41]

Fig. 6 (a)-(c) Schematic diagram of thinness- and shape- controlled growth and images of as-prepared CH3NH3PbI3 single crystal wafer; (d) Mass photodetectors based on a piece of single CH3NH3PbI3 crystal wafer[57]
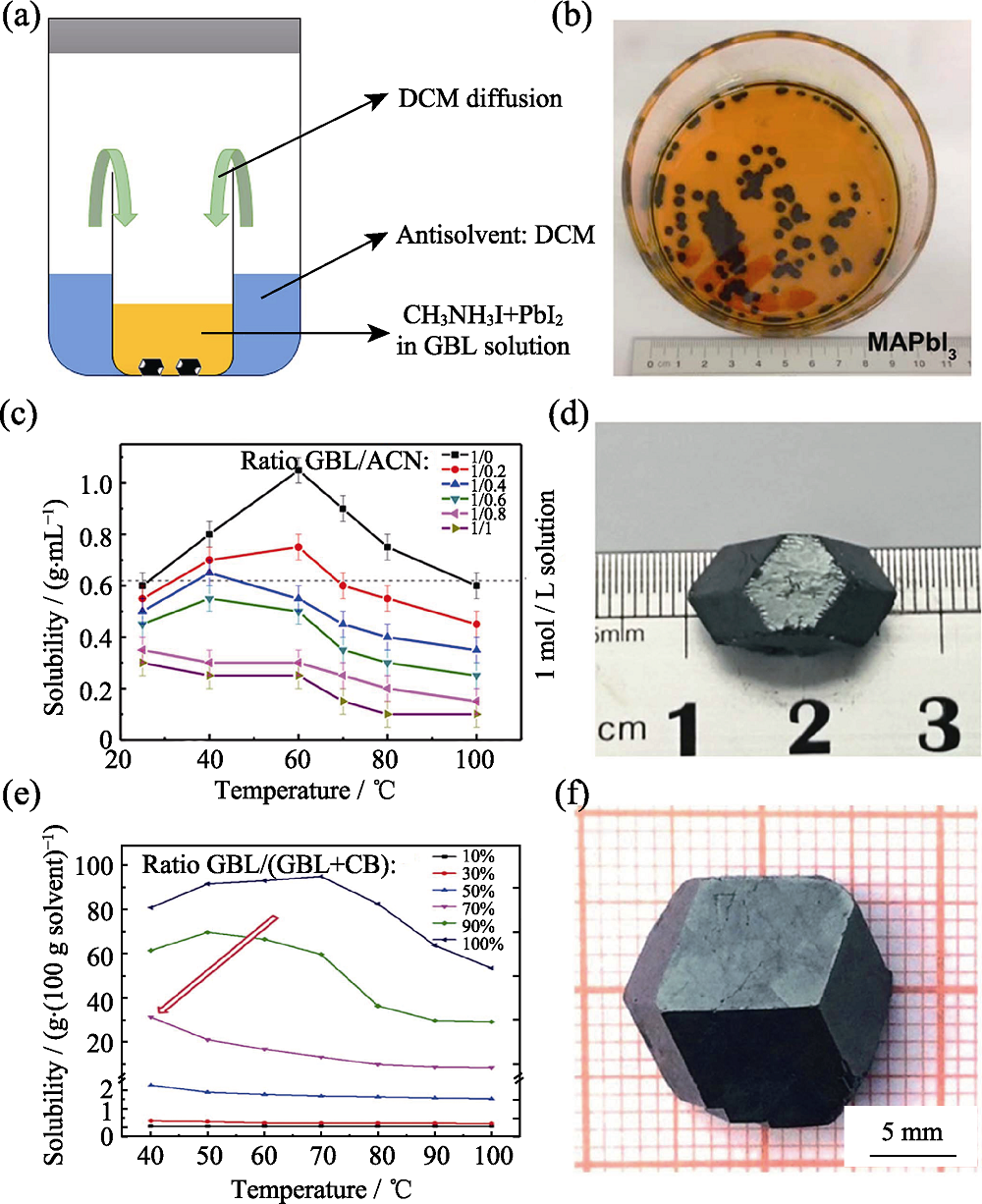
Fig. 7 Schematic diagram of solvent assisted crystallization (SAC) (a)-(b) Crystallization process of DCM assisted and images of as-prepared CH3NH3PbI3 crystals[39]; (c)-(d) Solubility of CH3NH3PbI3 at different temperatures in mixed-solvent of GBL and ACN, and image of as-prepared CH3NH3PbI3 single crystal[44]; (e)-(f) Solubility of CH3NH3PbI3 at different temperatures in mixed-solvent of GBL and CB, and image of as-prepared CH3NH3PbI3 single crystal[45]
| Growth method | Solvent | T/℃ | Size/mm | Carrier mobility/ (cm2•V-1•s-1) | Trap density/cm-3 | Bandgap/eV | Crystal system | Ref. |
|---|---|---|---|---|---|---|---|---|
| STL | HI | 65→40 | 10×10×8 | — | — | 1.48 | Tetragonal | [38] |
| HI | 100→57 | 12×12×7 | — | — | 1.48 | Tetragonal | [42] | |
| HI | 105→40 | 20×18×6 | 167±35 | (1.8±1.0)×109 | — | Tetragonal | [60] | |
| HI | 75 | 10×3 | 164±25 | 3.6×1010 | — | Tetragonal | [40] | |
| ITC | GBL | 60→110 | 5.8 | 67.2±7.3 | (1.4±0.2)×1010 | 1.51 | Tetragonal | [41] |
| GBL | 50→100 | 71×54×39 | 34 | 4.8×1010 | 1.53 | Tetragonal | [43] | |
| GBL | — | 113×58×52 | 41 | 2.1×108 | — | Tetragonal | [55] | |
| GBL | 60→110 | 150 μm in thickness | 39.6 | 6.0×108 | 1.45 | Tetragonal | [56] | |
| SAC | GBL/DCM | Room temperature | Millimeters | 2.5 | (3.3±0.3)×1010 | 1.51 | Tetragonal | [39] |
| GBL/ACN | 60→70 | 17 | — | — | — | Tetragonal | [44] | |
| GBL/CB | 30→60 | 15×15×10 | — | — | — | Cubic | [45] |
Table 1 A summary of properties of CH3NH3PbI3 single crystal by different methods
| Growth method | Solvent | T/℃ | Size/mm | Carrier mobility/ (cm2•V-1•s-1) | Trap density/cm-3 | Bandgap/eV | Crystal system | Ref. |
|---|---|---|---|---|---|---|---|---|
| STL | HI | 65→40 | 10×10×8 | — | — | 1.48 | Tetragonal | [38] |
| HI | 100→57 | 12×12×7 | — | — | 1.48 | Tetragonal | [42] | |
| HI | 105→40 | 20×18×6 | 167±35 | (1.8±1.0)×109 | — | Tetragonal | [60] | |
| HI | 75 | 10×3 | 164±25 | 3.6×1010 | — | Tetragonal | [40] | |
| ITC | GBL | 60→110 | 5.8 | 67.2±7.3 | (1.4±0.2)×1010 | 1.51 | Tetragonal | [41] |
| GBL | 50→100 | 71×54×39 | 34 | 4.8×1010 | 1.53 | Tetragonal | [43] | |
| GBL | — | 113×58×52 | 41 | 2.1×108 | — | Tetragonal | [55] | |
| GBL | 60→110 | 150 μm in thickness | 39.6 | 6.0×108 | 1.45 | Tetragonal | [56] | |
| SAC | GBL/DCM | Room temperature | Millimeters | 2.5 | (3.3±0.3)×1010 | 1.51 | Tetragonal | [39] |
| GBL/ACN | 60→70 | 17 | — | — | — | Tetragonal | [44] | |
| GBL/CB | 30→60 | 15×15×10 | — | — | — | Cubic | [45] |
| [1] | KOJIMA A, TESHIMA K, SHIRAI Y,et al. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J. Am. Chem. Soc., 2009, 131(17): 6050-6051. |
| [2] | YANG W S, PARK B W, JUNG E H,et al. Iodide management in formamidinium-lead-halide-based perovskite layers for efficient solar cells. Science, 2017, 356(6345): 1376-1379. |
| [3] | |
| [4] | WEBER D.CH3NH3PbX3, a Pb(II)-system with cubic perovskite structure.Z. Naturforsch., B: Anorg. Chem., Org. Chem., 1978, 33: 1443-1445. |
| [5] | MITZI D B.Templating and structural engineering in organic- inorganic perovskites.J. Chem. Soc., Dalton Trans., 2001, 1: 1-12. |
| [6] | SIDEY V.On the effective ionic radii for ammonium.Acta Crystallogr., Sect. B: Struct. Sci., 2016, 72: 626-633. |
| [7] | NIE W, TSAI H, ASADPOUR R,et al. High-efficiency solution- processed perovskite solar cells with millimeter-scale grains. Science, 2015, 347(6221): 522-525. |
| [8] | LIU C, FAN J, LI H,et al. Highly efficient perovskite solar cells with substantial reduction of lead content. Sci. Rep., 2016, 6: 35705. |
| [9] | WU Y Z, ISLAM A, YANG X D,et al. Retarding the crystallization of PbI2 for highly reproducible planar-structured perovskite solar cells via sequential deposition. Energy Environ. Sci., 2014, 7(9): 2934-2938. |
| [10] | MEI A Y, LI X, LIU L F,et al. A hole-conductor-free, fully printable mesoscopic perovskite solar cell with high stability. Science, 2014, 345(6194): 295-298. |
| [11] | SHI J, DONG J, LV S,et al. Hole-conductor-free perovskite organic lead iodide heterojunction thin-film solar cells: high efficiency and junction property. Appl. Phys. Lett., 2014, 104(6): 063901. |
| [12] | WU Y Z, YANG X D, CHEN W, et al. Perovskite solar cells with 18.21% efficiency Perovskite solar cells with 18.21% efficiency and area over 1 cm2 fabricated by heterojunction engineering. Nat. Energy, 2016, 1: 16148-1-7. |
| [13] | HU H, YAN K, PENG M,et al. Fiber-shaped perovskite solar cells with 5.3% efficiency. J. Mater. Chem. A, 2016, 4(10): 3901-3906. |
| [14] | YE T, FU W, WU J,et al. Single-crystalline lead halide perovskite arrays for solar cells. J. Mater. Chem. A, 2016, 4(4): 1214-1217. |
| [15] | YAN K, PENG M, YU X,et al. High-performance perovskite memristor based on methyl ammonium lead halides. J. Mater. Chem. C, 2016, 4(7): 1375-1381. |
| [16] | NIU G, LI W, MENG F,et al. Study on the stability of CH3NH3PbI3 films and the effect of post-modification by aluminum oxide in all-solid-state hybrid solar cells. J. Mater. Chem. A, 2013, 2(3): 705-710. |
| [17] | LIU C, DING W, ZHOU X,et al. Efficient and stable perovskite solar cells prepared in ambient air based on surface-modified perovskite layer. J. Phys. Chem. C, 2017, 121(12): 6546-6553. |
| [18] | CHATTERJEE S, PA A J.Introducing Cu2O thin films as a hole-transport layer in efficient planar perovskite solar cell structures.J. Phys. Chem. C, 2016, 120(3): 1428-1437. |
| [19] | XU W, YAO X, MENG T,et al. Perovskite hybrid solar cells with a fullerene derivative electron extraction layer. J. Mater. Chem. C, 2017, 5: 4190-4197. |
| [20] | SUN C, WU Z, YIP H L,et al. Amino-functionalized conjugated polymer as an efficient electron transport layer for high-performance planar-heterojunction perovskite solar cells. Adv. Energy Mater., 2016, 6(5): 1501534. |
| [21] | SU J, CHEN D P, LIN C T.Growth of large CH3NH3PbX3 (X=I, Br) single crystals in solution.J. Cryst. Growth, 2015, 422: 75-79. |
| [22] | ZHOU H, NIE Z, YIN J,et al. Antisolvent diffusion-induced growth, equilibrium behaviours in aqueous solution and optical properties of CH3NH3PbI3 single crystals for photovoltaic applications. RSC Adv., 2015, 5(104): 85344-85349. |
| [23] | RONG Y, TANG Z, ZHAO Y,et al. Solvent engineering towards controlled grain growth in perovskite planar heterojunction solar cells. Nanoscale, 2015, 7(24): 10595-10599. |
| [24] | HUANG J S, SHAO Y C, DONG Q F,et al. Organometal trihalide perovskite single crystals: a next wave of materials for 25% efficiency photovoltaics and applications beyond? J. Phys. Chem. Lett., 2015, 6(16): 3218-3227. |
| [25] | POGLITSCH A, WEBER D.Dynamic disorder in methylammoniumtrihalogenoplumbates (II) observed by millimeter-wave spectroscopy.J. Chem. Phys., 1987, 87(11): 6373-6378. |
| [26] | BAIKIE T, FANG Y, KADRO J M,et al. Synthesis and crystal chemistry of the hybrid perovskite (CH3NH3)PbI3 for solid-state sensitised solar cell applications. J. Mater. Chem. A, 2013, 1(18): 5628-5641. |
| [27] | IM J H, LEE C R, LEE J W,et al. 6.5% Efficient perovskite quantum- dot-sensitized solar cell. Nanoscale, 2011, 3(10): 4088-4093. |
| [28] | LEE M M, TEUSCHER J, MIYASAKA T,et al. Efficient hybrid solar cells based on meso-superstructured organometal halide perovskites. Science, 2012, 338(6107): 643-647. |
| [29] | KIM H S, LEE C R, IM J H,et al. Lead iodide perovskite sensitized all-solid-state submicron thin film mesoscopic solar cell with efficiency exceeding 9%. Sci. Rep., 2012, 2: 591. |
| [30] | BURSHKA J, PELLET N, MOON S J,et al. Sequential deposition as a route to high-performance perovskite-sensitized solar cells. Nature, 2013, 499(7458): 316-319. |
| [31] | KU Z, RONG Y, XU M,et al. Full printable processed mesoscopic CH3NH3PbI3/TiO2 heterojunction solar cells with carbon counter electrode. Sci. Rep., 2013, 3: 3132. |
| [32] | LIU M Z, JOHNSTON M B, SNAITH H J,et al. Efficient planar heterojunction perovskite solar cells by vapour deposition. Nature, 2013, 501(7467): 395-398. |
| [33] | ZHOU H, CHEN Q, LI G,et al. Interface engineering of highly efficient perovskite solar cells. Science, 2014, 345(6196): 542-546. |
| [34] | ZHANG W, PATHAK S, SAKAI N, et al. Enhanced optoelectronic quality of perovskite thin films with hypophosphorous acid for planar heterojunction solar cells. Nat. Commun , 2015, 6: 10030-1-9. |
| [35] | YANG W S, NOH J H, JEON N J,et al. High-performance photovoltaic perovskite layers fabricated through intramolecular exchange. Science, 2015, 348(6240): 1234-1237. |
| [36] | STOUMPOS C C, MALLIAKAS C D, KANATZIDIS M G,et al. Semiconducting tin and lead iodide perovskites with organic cations: phase transitions, high mobilities, and near-infrared photoluminescent properties. Inorg. Chem., 2013, 52(15): 9019-9038. |
| [37] | PISONI A, JACIMOVIC J, BARISIC O S,et al. Ultra-low thermal conductivity in organic-inorganic hybrid perovskite CH3NH3PbI3. J. Phys. Chem. Lett., 2014, 5(14): 2488-2492. |
| [38] | DANG Y, LIU Y, SUN Y,et al. Bulk crystal growth of hybrid perovskite material CH3NH3PbI3. CrystEngComm, 2015, 17(3): 665-670. |
| [39] | SHI D, ADINOLFI V, COMIN R,et al. Low trap-state density and long carrier diffusion in organolead trihalide perovskite single crystals. Science, 2015, 347(6221): 519-522. |
| [40] | DONG Q, FANG Y, SHAO Y,et al. Electron-hole diffusion lengths > 175 μm in solution-grown CH3NH3PbI3 single crystals. Science, 2015, 347(6225): 967-970. |
| [41] | SAIDAMINOV M I, ABDELHADY A L, MURALI B, et al. High-quality bulk hybrid perovskite single crystals within minutes by inverse temperature crystallization. Nat. Commun., 2015, 6: 7586-1-6. |
| [42] | LIAN Z, YAN Q, LV Q,et al. High-performance planar-type photodetector on (100) facet of MAPbI3 single crystal. Sci. Rep., 2015, 5: 16563. |
| [43] | LIU Y, YANG Z, CUI D,et al. Two-inch-sized perovskite CH3NH3PbX3(X = Cl, Br, I) crystals: growth and characterization. Adv. Mater., 2015, 27(35): 5176-5183. |
| [44] | KU Z, TIEP N H, WU B,et al. Solvent engineering for fast growth of centimetric high-quality CH3NH3PbI3perovskite single crystals. New J. Chem., 2016, 40(9): 7261-7264. |
| [45] | LUAN M, SONG J, WEI X,et al. Controllable growth of bulk cubic-phase CH3NH3PbI3 single crystal with exciting room temperature stability. CrystEngComm, 2016, 18(28): 5257-5261. |
| [46] | LI G.Preparation and Characterization of Organic-inorganic Hybrid Perovskite Materials. Changsha: National University of Defense Technology Bachelor Dissertation, 2016. |
| [47] | XIAO Z, DONG Q, BI C,et al. Solvent annealing of perovskite-induced crystal growth for photovoltaic-device efficiency enhancement. Adv. Mater., 2014, 26(37): 6503-6509. |
| [48] | DONG Q, SONG J, FANG Y,et al. Lateral-structure single-crystal hybrid perovskite solar cells via piezoelectric poling. Adv. Mater., 2016, 28(14): 2816-2821. |
| [49] | ZHOU Y Y, LI C M, WANG Y, et al. Preparation and Characterization of High-quality Perovskite CH3NH3PbX3(I, Br) Single Crystal. 1st International Conference on New Material and Chemical Industry, SanYa, 2017, 167: 012019. |
| [50] | QIN X, YAO Y, DONG H,et al. Perovskite photodetectors based on CH3NH3PbI3 single crystals. Chem. Asian J., 2016, 11(19): 2675-2679. |
| [51] | DANG Y, JU D, WANG L,et al. Recent progress in the synthesis of hybrid halide perovskite single crystals. CrystEngComm, 2016, 18(24): 4476-4484. |
| [52] | LEGUY A M A, HU Y, CAMPOY M,et al. Reversible hydration of CH3NH3PbI3in films, single crystals, and solar cells. Chem. Mater., 2015, 27(9): 3397-3407. |
| [53] | VINCENT B R, ROBERTSON K N, CAMERON T S,et al. Alkylammonium lead halides. Part 1. Isolated PbI64- ions in (CH3NH3)4PbI6·2H2O. Can. J. Chem., 1987, 65: 1042-1046. |
| [54] | KADRO J M, NONOMURA K, GACHET D,et al. Facile route to freestanding CH3NH3PbI3 crystals using inverse solubility. Sci. Rep., 2015, 5: 11654. |
| [55] | KATZ E A.High quality large single crystals of metal halide perovskites for optoelectronic applications.Sci. Chi. Chem., 2017, 60(10): 1326-1328. |
| [56] | LIU Y C, REN X D, ZHANG J,et al. 120 millimeter single-crystalline perovskite and wafers: towards viable applications. Sci. Chi. Chem., 2017, 60(10): 1367-1376. |
| [57] | LIU Y, ZHANG Y, YANG Z,et al. Thinness- and shape-controlled growth for ultrathin single-crystalline perovskite wafers for mass production of superior photoelectronic devices. Adv. Mater., 2016, 28(41): 9204-9209. |
| [58] | NAYAK P K, MOORE D T, WENGER B,et al. Mechanism for rapid growth of organic-inorganic halide perovskite crystals. Nat. Commun., 2016, 7: 13303. |
| [59] | KAWAMURA Y, MASHIYAMA H, HASEBE, K. structural study on cubic-tetragonal transition of CH3NH3PbI3.J. Phys. Soc. Jpn., 2002, 71(7): 1694-1697. |
| [60] | LIAN Z, YAN Q, GAO T,et al. Perovskite CH3NH3PbI3(Cl) single crystals: rapid solution growth, unparalleled crystalline quality, and low trap density toward 108 cm-3. J. Am. Chem. Soc., 2016, 138(30): 9409-9412. |
| [61] | YANG B, KEUM J, OVCHINNIKOVA O S,et al. Deciphering halogen competition in organometallic halide perovskite growth. J. Am. Chem. Soc., 2016, 138(15): 5028-5035. |
| [62] | ZHANG Y, HUANG F Q, MI Q X,et al. Preferential facet growth of methylammonium lead halide single crystals promoted by halide coordination. Chem. Lett., 2016, 45(8): 1030-1032. |
| [63] | DING J, DU S, ZHAO Y,et al. High-quality inorganic-organic perovskite CH3NH3PbI3 single crystals for photo-detector applications. J. Mater. Sci., 2017, 52(1): 276-284. |
| [64] | XING G, MATHEWS N, LIM S S,et al. Low-temperature solution- processed wavelength-tunable perovskites for lasing. Nat. Mater., 2014, 13(5): 476-480. |
| [65] | DOU L, YANG Y, YOU J, et al. Solution-processed hybrid perovskite photodetectors with high detectivity. Nat. Commun., 2014, 5: 5404-1-6. |
| [66] | WEI H, FANG Y, MULLIGAN P,et al. Sensitive X-ray detectors made of methylammonium lead tribromide perovskite single crystals. Nature Photon., 2016, 10: 333-339. |
| [67] | TAN Z K, MOGHADDAM R S, LAI M L,et al. Bright light-emitting diodes based on organometal halide perovskite. Nature Nanotech., 2014, 9(9): 687-692. |
| [68] | STRANKS S D, EPERON G E, GRANCINI G,et al. Electron-hole diffusion lengths exceeding 1 micrometer in an organometal trihalide perovskite absorber. Science, 2013, 342(6156): 341-344. |
| [69] | KIM H S, MORA-SERO I, GONZALEZ V,et al. Mechanism of carrier accumulation in perovskite thin-absorber solar cells. Nat. Commun., 2013, 4: 2242. |
| [70] | CHEN Y S, MANSER J S, KAMAT P V.All solution-processed lead halide perovskite-BiVO4 tandem assembly for photolytic solar fuels production.J. Am. Chem. Soc., 2015, 137(2): 974-981. |
| [71] | XU Q, WEI H, WEI W,et al. Detection of charged particles with a methylammonium lead tribromide perovskite single crystal. Nucl. Instrum. Methods Phys. Res., Sect. A, 2017, 848: 106-108. |
| [72] | SEMONIN O E, ELBAZ G A, STRAUS D B,et al. Limits of carrier diffusion in n-type and p-type CH3NH3PbI3 perovskite single crystals. J. Phys. Chem. Lett., 2016, 7(17): 3510-3518. |
| [73] | WANGYANG P H, SUN H, ZHU X H, et al.Solution-processable methyl ammonium lead iodide single crystal photodetectors for visible light and X-ray. Phys. Status Solidi A, 2017, 214(11): 1700538-1-5. |
| [74] | DING J, YAN Q F.Progress in organic-inorganic hybrid halide perovskite single crystal: growth techniques and applications.Sci. Chi. Mater., 2017, 60: 1063-1078. |
| [1] | DING Ling, JIANG Rui, TANG Zilong, YANG Yunqiong. MXene: Nanoengineering and Application as Electrode Materials for Supercapacitors [J]. Journal of Inorganic Materials, 2023, 38(6): 619-633. |
| [2] | YANG Zhuo, LU Yong, ZHAO Qing, CHEN Jun. X-ray Diffraction Rietveld Refinement and Its Application in Cathode Materials for Lithium-ion Batteries [J]. Journal of Inorganic Materials, 2023, 38(6): 589-605. |
| [3] | CHEN Qiang, BAI Shuxin, YE Yicong. Highly Thermal Conductive Silicon Carbide Ceramics Matrix Composites for Thermal Management: a Review [J]. Journal of Inorganic Materials, 2023, 38(6): 634-646. |
| [4] | LIN Junliang, WANG Zhanjie. Research Progress on Ferroelectric Superlattices [J]. Journal of Inorganic Materials, 2023, 38(6): 606-618. |
| [5] | ZHANG Shouchao, CHEN Hongyu, LIU Hongfei, YANG Yu, LI Xin, LIU Defeng. High Temperature Recovery of Neutron Irradiation-induced Swelling and Optical Property of 6H-SiC [J]. Journal of Inorganic Materials, 2023, 38(6): 678-686. |
| [6] | NIU Jiaxue, SUN Si, LIU Pengfei, ZHANG Xiaodong, MU Xiaoyu. Copper-based Nanozymes: Properties and Applications in Biomedicine [J]. Journal of Inorganic Materials, 2023, 38(5): 489-502. |
| [7] | YUAN Jingkun, XIONG Shufeng, CHEN Zhangwei. Research Trends and Challenges of Additive Manufacturing of Polymer-derived Ceramics [J]. Journal of Inorganic Materials, 2023, 38(5): 477-488. |
| [8] | YOU Junqi, LI Ce, YANG Dongliang, SUN Linfeng. Double Dielectric Layer Metal-oxide Memristor: Design and Applications [J]. Journal of Inorganic Materials, 2023, 38(4): 387-398. |
| [9] | DU Jianyu, GE Chen. Recent Progress in Optoelectronic Artificial Synapse Devices [J]. Journal of Inorganic Materials, 2023, 38(4): 378-386. |
| [10] | YANG Yang, CUI Hangyuan, ZHU Ying, WAN Changjin, WAN Qing. Research Progress of Flexible Neuromorphic Transistors [J]. Journal of Inorganic Materials, 2023, 38(4): 367-377. |
| [11] | LI Yicun, LIU Xuedong, HAO Xiaobin, DAI Bing, LYU Jilei, ZHU Jiaqi. Rapid Growth of Single Crystal Diamond at High Energy Density by Plasma Focusing [J]. Journal of Inorganic Materials, 2023, 38(3): 303-309. |
| [12] | QI Zhanguo, LIU Lei, WANG Shouzhi, WANG Guogong, YU Jiaoxian, WANG Zhongxin, DUAN Xiulan, XU Xiangang, ZHANG Lei. Progress in GaN Single Crystals: HVPE Growth and Doping [J]. Journal of Inorganic Materials, 2023, 38(3): 243-255. |
| [13] | WANG Zhiqiang, WU Ji’an, CHEN Kunfeng, XUE Dongfeng. Large-size Er,Yb:YAG Single Crystal: Growth and Performance [J]. Journal of Inorganic Materials, 2023, 38(3): 329-334. |
| [14] | ZHANG Chaoyi, TANG Huili, LI Xianke, WANG Qingguo, LUO Ping, WU Feng, ZHANG Chenbo, XUE Yanyan, XU Jun, HAN Jianfeng, LU Zhanwen. Research Progress of ScAlMgO4 Crystal: a Novel GaN and ZnO Substrate [J]. Journal of Inorganic Materials, 2023, 38(3): 228-242. |
| [15] | CHEN Kunfeng, HU Qianyu, LIU Feng, XUE Dongfeng. Multi-scale Crystallization Materials: Advances in in-situ Characterization Techniques and Computational Simulations [J]. Journal of Inorganic Materials, 2023, 38(3): 256-269. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||