
Journal of Inorganic Materials ›› 2018, Vol. 33 ›› Issue (3): 352-356.DOI: 10.15541/jim20170163
• Orginal Article • Previous Articles Next Articles
YUAN Bei-Bei1, 2, 3, ZHOU Bei-Bei1, 2, 3, ZHANG Yue-Biao1, 3, SHI Jian-Lin1, 2, 3
Received:2017-04-07
Published:2018-03-20
Online:2018-03-12
About author:YUAN Bei-Bei (1991-), female, candidate of Master degree. E-mail: yuanbb@shanghaitech.edu.cn
Supported by:CLC Number:
YUAN Bei-Bei, ZHOU Bei-Bei, ZHANG Yue-Biao, SHI Jian-Lin. Charge-switchable Metal-organic Framework for Size/Charge-selective Molecular Inclusions[J]. Journal of Inorganic Materials, 2018, 33(3): 352-356.

Fig. S2 (a) The rhombicuboctahedral cage enclosed alternatively by eight Zn2(COO)3+ and six Zn2(COO)4 clusters; (b) The cuboctahedral cage enclosed by four Zn2(COO)3+ clusters; (c) The truncated octahedral cage enclosed by six Zn2(COO)4 clusters; (d) The square-bifrustum cage enclosed alternatively by four Zn2(COO)3+ clusters and four Zn2(COO)4 clusters.The open pores size of cages in (a), (b), (c) and (d) are (e) 0.40 × 0.60 nm, (f) 0.72 × 0.76 nm, (g) 1.04 × 0.34 nm and (h) 0.70 × 0.90 nm, respectively
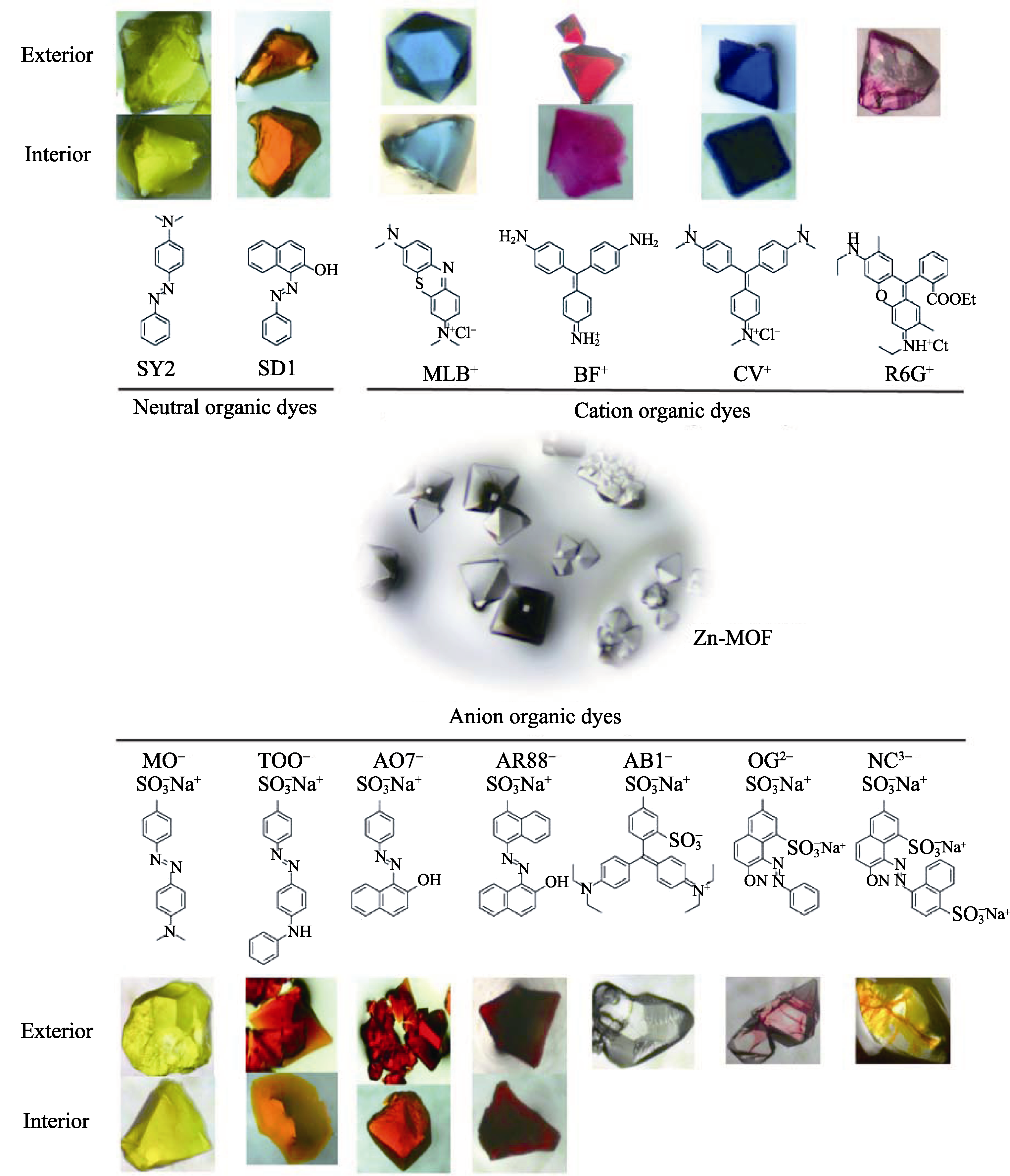
Fig. S3 The optical microscope images of the Zn-MOF crystals before (centered) and after (top and bottom) dye inclusion experiments. To confirm the uptake homogeneity of dyes, both the exterior and interior of dyes adsorbed MOF crystals were shown except those for AB-, OG2-, and NC3- with dyes penetrated only the outer part of the crystals
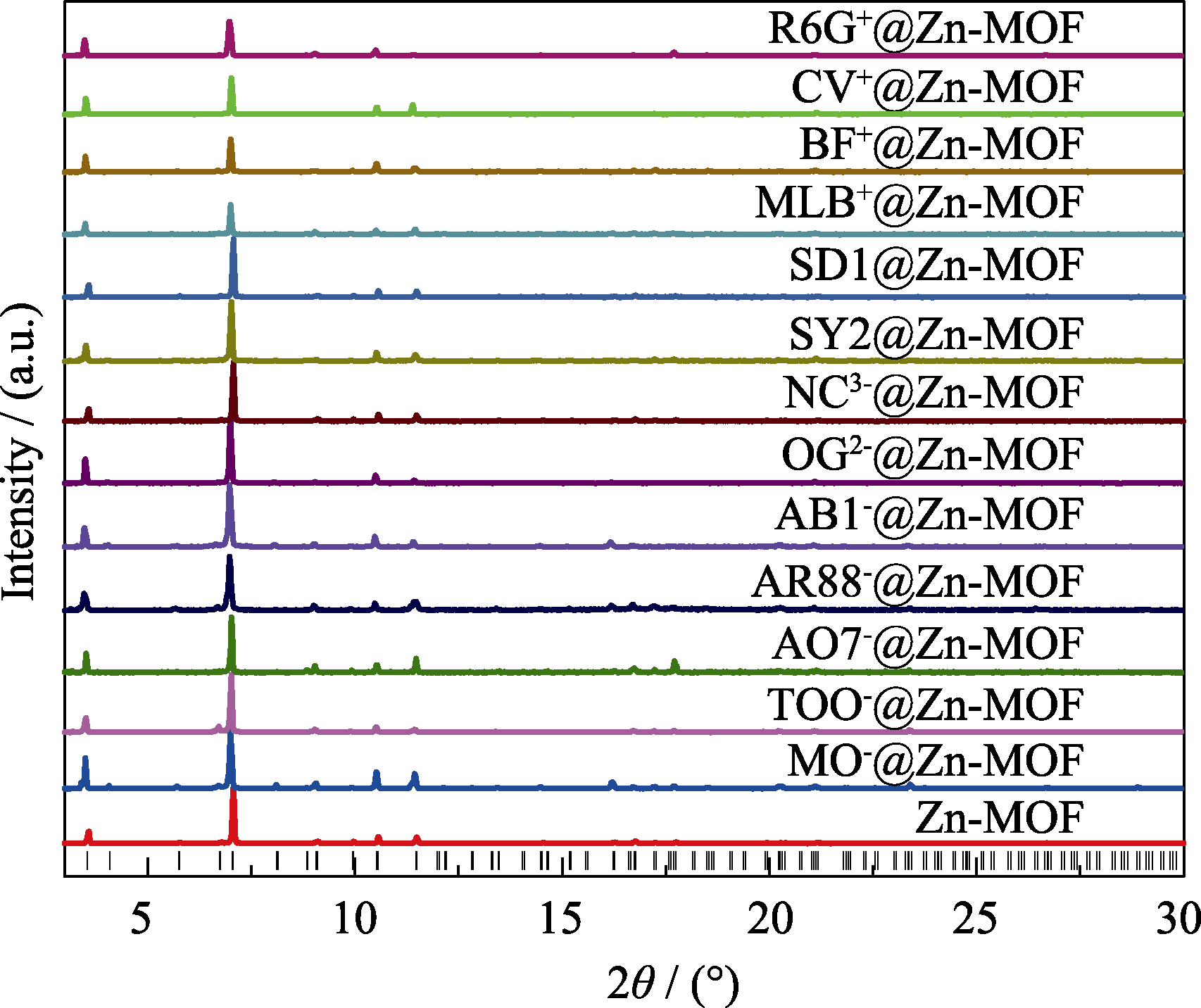
Fig. S4 PXRD patterns of Zn-MOF and dye@Zn-MOF samples compared with simulation All the PXRD were carried out with a few drop of DMF on the surface. It shows that the crystal structure is still maintained after adsorption of dyes
| MOFs | Absorption capacity/(mg•g-1) | Metal | Framework charge | Ref. | |
|---|---|---|---|---|---|
| Cationic dye | Anionic dye | ||||
| MOF-5 | Congo Red | NM | Zn | Neutral | [1] |
| MOF-5 | Pyronin Y, Azure A | NM | Zn | Neutral | [2] |
| ZIF-8 | 9.2 (MLB) | ~11.6 (MO) | Zn | Neutral | [3] |
| ZIF-8 | 5.4 (MLB) | 22 (AB40) | Zn | Neutral | [4] |
| IFMC-2 | MLB, CV | NA | Zn | Anionic | [5] |
| Compound 1 | MLB, RhB | NA | Zn | Anionic | [6] |
| NENU-505 | MLB, BR2 | NA | Zn | Anionic | [7] |
| Zn-MOF | 12.6 (MLB) | 19 (MO) | Zn | Cationic | This work |
| ITC-4 | NA | 77.4 (OG) | In | Cationic | [8] |
| Compound 1 | NA | 183.5 (OG) | In | Cationic | [9] |
| MOF-235 | 252.0 (MLB) | 477.0 (MO) | Fe | Cationic | [10] |
| MIL-100(Fe) | 736.2 (MLB) | 1045.2 (MO) | Fe | Cationic | [11] |
| MIL-100(Cr) | 643.3 (MLB) | 211.8 (MO) | Cr | Cationic | [11] |
| PCN-222 | 906 (MLB) | 589 (MO) | Zr | Neutral | [12] |
Table S2 The adsorption capacity of selected MOFs and Zn-MOF toward MLB and MO
| MOFs | Absorption capacity/(mg•g-1) | Metal | Framework charge | Ref. | |
|---|---|---|---|---|---|
| Cationic dye | Anionic dye | ||||
| MOF-5 | Congo Red | NM | Zn | Neutral | [1] |
| MOF-5 | Pyronin Y, Azure A | NM | Zn | Neutral | [2] |
| ZIF-8 | 9.2 (MLB) | ~11.6 (MO) | Zn | Neutral | [3] |
| ZIF-8 | 5.4 (MLB) | 22 (AB40) | Zn | Neutral | [4] |
| IFMC-2 | MLB, CV | NA | Zn | Anionic | [5] |
| Compound 1 | MLB, RhB | NA | Zn | Anionic | [6] |
| NENU-505 | MLB, BR2 | NA | Zn | Anionic | [7] |
| Zn-MOF | 12.6 (MLB) | 19 (MO) | Zn | Cationic | This work |
| ITC-4 | NA | 77.4 (OG) | In | Cationic | [8] |
| Compound 1 | NA | 183.5 (OG) | In | Cationic | [9] |
| MOF-235 | 252.0 (MLB) | 477.0 (MO) | Fe | Cationic | [10] |
| MIL-100(Fe) | 736.2 (MLB) | 1045.2 (MO) | Fe | Cationic | [11] |
| MIL-100(Cr) | 643.3 (MLB) | 211.8 (MO) | Cr | Cationic | [11] |
| PCN-222 | 906 (MLB) | 589 (MO) | Zr | Neutral | [12] |
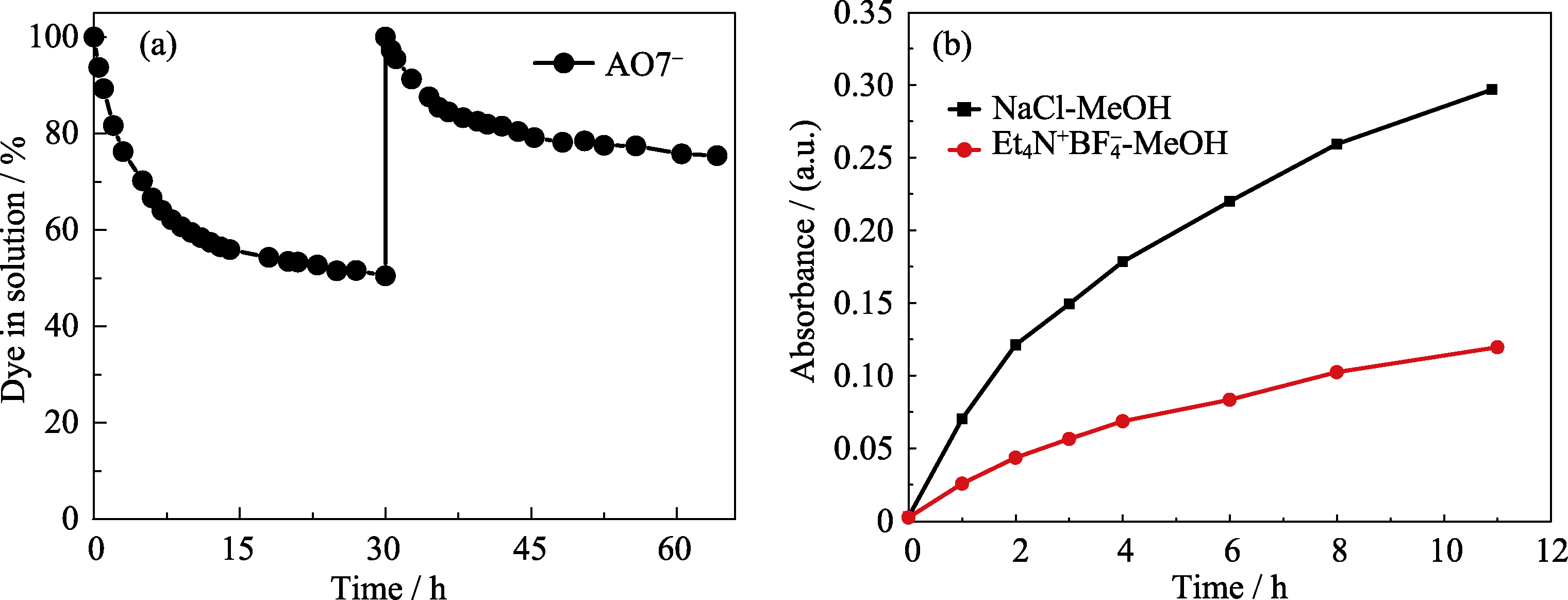
Fig. 1 (a) Dye adsorption kinetics of AO7- by Zn-MOF, which was immersed into a fresh AO7- solution at 30 h for second uptake, indicating that the concentration gradient potential served as the driving force for dye inclusion; (b) Release rates of AR88- (at λmax = 509 nm) salt out from AR88-@Zn-MOF in 0.18 mol/L methanol solution of NaCl and Et4N+BF4-, respectively
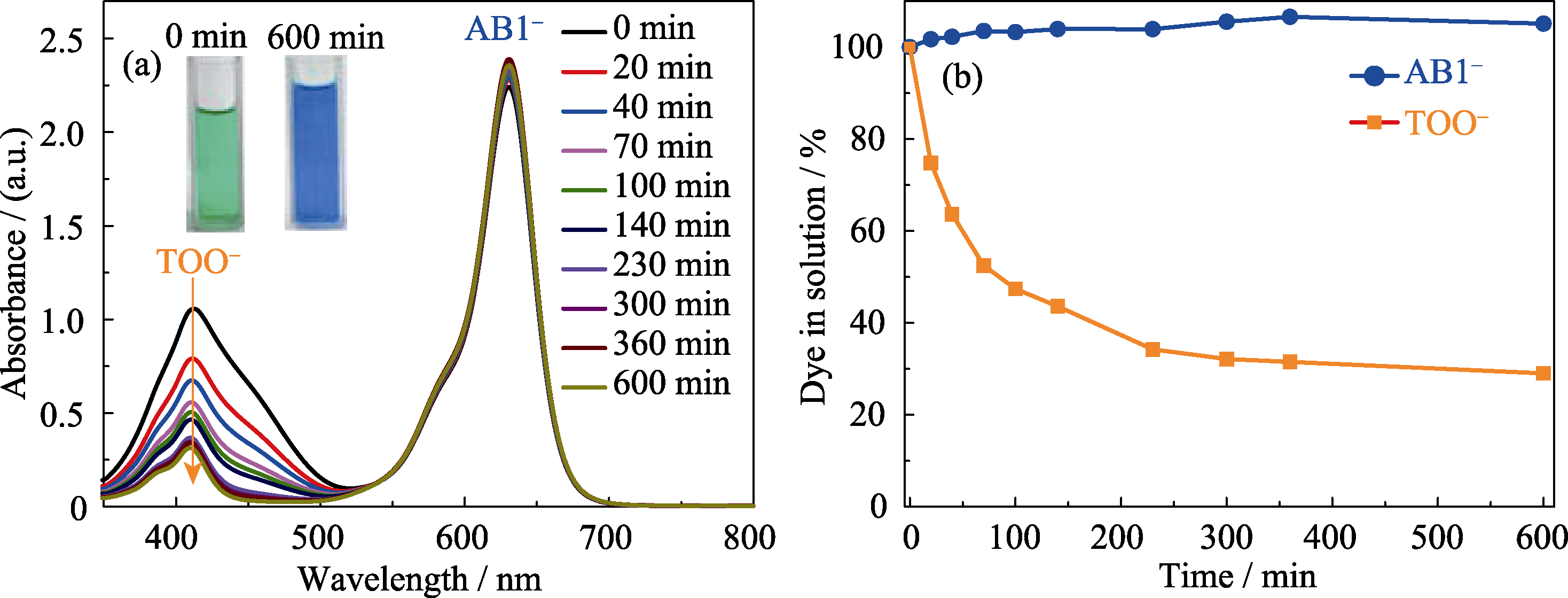
Fig. 2 (a) The time dependent UV-Vis spectra of the solutions of AB1- and TOO- in the ratio of 765∶1 for the competitive adsorption by Zn-MOF (λmax= 412 nm for TOO-, λmax= 630 nm for AB1-) with inset showing the colour of TOO-/AB1- solution before and after dye inclusion for 600 min, respectively; (b) The adsorption kinetics of AB1- and TOO- by Zn-MOF in the binary mixture solution
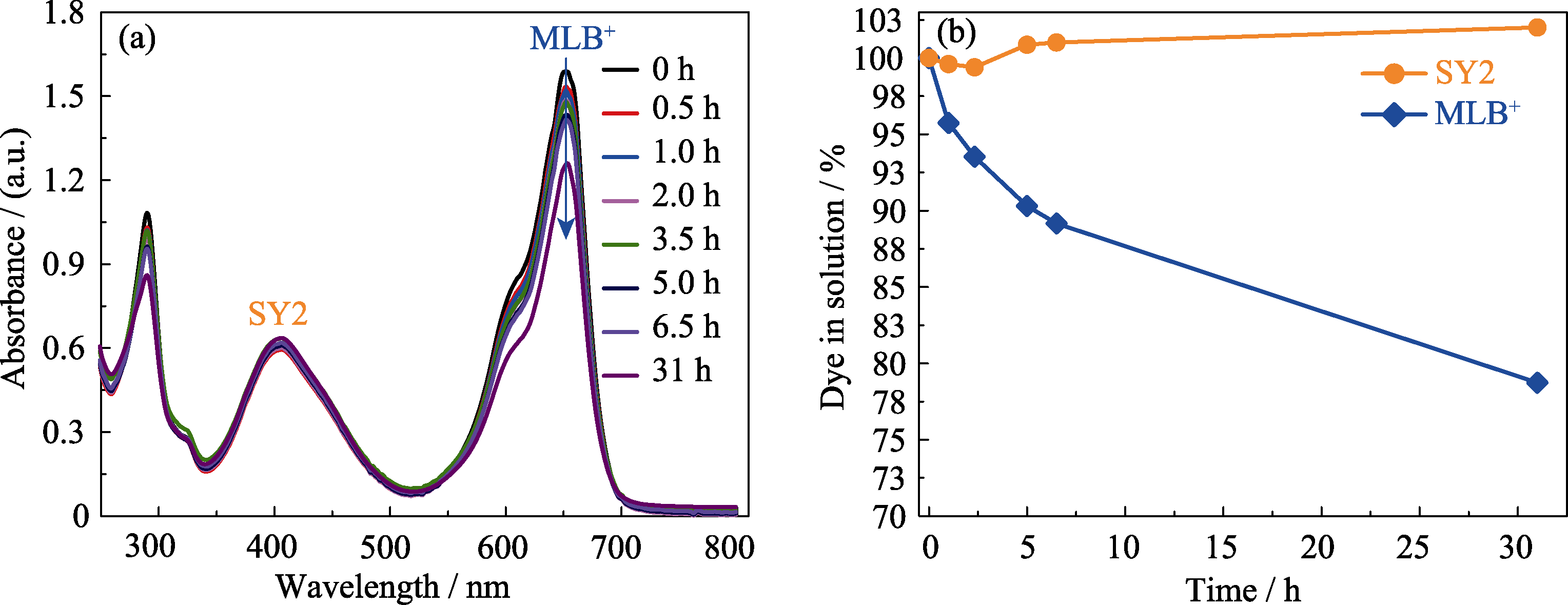
Fig. 4 (a) The time dependent UV-Vis spectra of the solutions of MLB+ and SY2 at the ratio of 1∶1 for the competitive adsorption by Zn-MOF from 0 to 31 h (λmax = 652 nm for MlB+, λmax = 406 nm for SY2); (b) The competitive adsorption rate of MIB+ and SY2 by the Zn-MOF
| [1] | CUI YUAN-JING, SONG RUI-JING, YU JIAN-CAN, et al.Dual-emitting MOF superset of dye composite for ratiometric temperature sensing. Adv. Mater., 2015, 27(8): 1420-1425. |
| [2] | XIE WEI, HE WEN-WEN, LI SHUN-LI, et al.An anionic interpenetrated zeolite-like metal-organic framework composite as a tunable dual-emission luminescent switch for detecting volatile organic molecules. Chem. Eur. J., 2016, 22(48): 17298-17304. |
| [3] | LAN YA-QIAN, JIANG HAI-LONG, LI SHUN-LI, et al.Mesoporous metal-organic frameworks with size-tunable cages: selective CO2 uptake, encapsulation of Ln3+ cations for luminescence, and column-chromatographic dye separation. Adv.Mater., 23(43): 5015-5020. |
| [4] | LI PEI-ZHOU, WANG XIAO-JUN, TAN SI-YU, et al.Clicked isoreticular metal-organic frameworks and their high performance in the selective capture and separation of large organic molecules. Angew. Chem., Int. Ed., 2015, 54(43): 12748-12752. |
| [5] | MA LI-QING, FALKOWSKI JOSEPH M, ABNEY CARTER, et al.A series of isoreticular chiral metal-organic frameworks as a tunable platform for asymmetric catalysis. Nat. Chem., 2010, 2(10): 838-846. |
| [6] | DELLA ROCCA JOSEPH, LIU DE-MIN, LIN WEN-BIN.Nanoscale metal-organic frameworks for biomedical imaging and drug delivery. Acc. Chem. Res., 2011, 44(10): 957-968. |
| [7] | ZHAO XIANG, MAO CHENG-YU, LUONG KAREN TU, et al.Framework cationization by preemptive coordination of open metal sites for anion-exchange encapsulation of nucleotides and coenzymes. Angew. Chem. Int. Ed., 2016, 55(8): 2768-2772. |
| [8] | LIU FEI, CHUNG SO-YI, OH GAHEE, et al.Three-dimensional graphene oxide nanostructure for fast and efficient water-soluble dye removal. ACS Appl. Mater. Interfaces, 2012, 4(2): 922-927. |
| [9] | SIMON V, THURET A, CANDY L, et al.Recovery of hydroxycinnamic acids from renewable resources by adsorption on zeolites. Chem. Eng. J., 2015, 280: 748-754. |
| [10] | ZHAO XIANG, BU XIAN-HUI, WU TAO, et al.Selective anion exchange with nanogated isoreticular positive metal-organic frameworks. Nat. Commun., 2013, 4: 2344-2348. |
| [11] | HASAN ZUBAIR, JHUNG SUNG HWA.Removal of hazardous organics from water using metal-organic frameworks (MOFs): plausible mechanisms for selective adsorptions. J. Hazard. Mater., 2015, 283: 329-339. |
| [12] | HAN YI, SHENG SHU-NAN, YANG FAN, et al.Size-exclusive and coordination-induced selective dye adsorption in a nanotubular metal-organic framework. J. Mater. Chem. A, 2015, 3(24): 12804-12809. |
| [13] | HAQUE ENAMUL, JUN JONG WON, JHUNG SUNG HWA.Adsorptive removal of methyl orange and methylene blue from aqueous solution with a metal-organic framework material, iron terephthalate (MOF-235). J. Hazard. Mater., 2011, 185(1): 507-511. |
| [14] | HAN SHUANG-BING, WEI YAN-HU, CORY VALENTE, et al.Chromatography in a single metal-organic framework (MOF) crystal. J. Am. Chem. Soc., 2010, 132(46): 16358-16361. |
| [15] | KANG XIAO-ZHEN, SONG ZHENG-WEI, SHI QI, et al.Utilization of zeolite imidazolate framework as an adsorbent for the removal of dye from aqueous solution. Asian [J]. Chem., 2013, 25(15): 8324-8328. |
| [16] | LI YU, ZHOU KANG, HE MING, et al.Synthesis of ZIF-8 and ZIF-67 using mixed-base and their dye adsorption. Microporous Mesoporous Mater., 2016, 234(1): 287-292. |
| [17] | QIN JUN-SHENG, ZHANG SHU-RAN, DU DONG-YING, et al.A microporous anionic metal-organic framework for sensing luminescence of lanthanide (III) ions and selective absorption of dyes by ionic exchange. Chem. Eur. J., 2014, 20(19): 5625-5630. |
| [18] | SHEN XIANG, YAN BING.Anionic metal-organic framework hybrids functionalization with lanthanide ions or cationic dyes and fluorescence sensing of small molecules. RSC Adv., 2016, 6(34): 28165-28170. |
| [19] | WU MING-YAN, JIANG FEI-LONG, WEI WEI, et al.A porous polyhedral metal-organic framework based on Zn2(COO)3 and Zn2, 2009, 9(6): 2559-2561. |
| [20] | LI HAI-CHAO, CAO XIN-YU, ZHANG CHUANG, et al.Enhanced adsorptive removal of anionic and cationic dyes from single or mixed dye solutions using MOF PCN-222. RSC Adv., 2017, 7(27): 16273-16281. |
| [21] | TONG MIN-MAN, LIU DA-HUAN, YANG QING-YUAN, et al.Influence of framework metal ions on the dye capture behavior of MIL-100 (Fe, Cr) MOF type solids. J. Mater. Chem. A, 2013, 1(30): 8534-8537. |
| [22] | KHANJANI SOMAYEH, MORSALI ALI.Ultrasound-promoted coating of MOF-5 on silk fiber and study of adsorptive removal and recovery of hazardous anionic dye “congo red’’. Ultrason. Sonochem. 2014, 21(4): 1424-1429. |
| [23] | HAN SHUANG-BING, WEI YAN-HU, CORY VALENTE, et al.Chromatography in a single metal-organic framework (MOF) crystal. J. Am. Chem. Soc., 2010, 132(46): 16358-16361. |
| [24] | LI YU, ZHOU KANG, HE MING, et al.Synthesis of ZIF-8 and ZIF-67 using mixed-base and their dye adsorption. Microporous Mesoporous Mater., 2016, 234(1): 287-292. |
| [25] | KANG XIAO-ZHEN, SONG ZHENG-WEI, SHI QI, et al.Utilization of zeolite imidazolate framework as an adsorbent for the removal of dye from aqueous solution. Asian [J]. Chem., 2013, 25(15): 8324-8328. |
| [26] | QIN JUN-SHENG, ZHANG SHU-RAN, DU DONG-YING, et al.A microporous anionic metal-organic Framework for sensing luminescence of lanthanide (III) ions and selective absorption of dyes by ionic exchange. Chem. Eur. J., 2014, 20(19): 5625-5630. |
| [27] | SHEN XIANG, YAN BING.Anionic metal-organic framework hybrids functionalization with lanthanide ions or cationic dyes and fluorescence sensing of small molecules. RSC Adv., 2016, 6(34): 28165-28170. |
| [28] | ZHANG SHU-RAN, LI JING, DU DONG-YING, et al.A multifunctional microporous anionic metal-organic framework for column-chromatographicdye separation and selective detection andadsorption of Cr3+. J. Mater. Chem. A, 2015, 3(46): 23426-23434. |
| [29] | ZHAO XIANG, BU XIAN-HUI, WU TAO, et al.Selective anion exchange with nanogated isoreticular positive metal-organic frameworks. Nat.Commun., 2013(4): 2344-2348. |
| [30] | SONG BAI-QIAO, WANG XIN-LONG, ZHANG YU-TENG, et al.Periodic tiling of triangular and square nanotubes in a cationic metal-organic framework for selective anion exchange. Chem. Commun., 2015, 51(46): 9515-9518. |
| [31] | HAQUE ENAMUL, JUN JONGWON, JHUNG SUNGHWA.Adsorptive removal of methyl orange and methylene blue from aqueous solution with a metal-organic framework material, iron terephthalate (MOF-235). J. Hazard. Mater., 2011, 185(1): 507-511. |
| [32] | TONG MIN-MAN, LIU DA-HUAN, YANG QING-YUAN, et al.Influence of framework metal ions on the dye capture behaviour of MIL-100(Fe, Cr) MOF type solids. J. Mater. Chem. A, 2013, 1(30): 8534-8537. |
| [33] | LI HAI-CHAO, CAO XIN-YU, ZHANG CHUANG, et al.Enhanced adsorptive removal of anionic and cationic dyes from single or mixed dye solutions using MOF PCN-222. RSC Adv., 2017, 7(27): 16273-16281. |
| [1] | GUO Lina, HE Xuebing, LYU Lin, WU Dan, YUAN Hong. Modulation of CuO Surface Properties for Selective Electrocatalytic Reduction of CO2 to HCOOH [J]. Journal of Inorganic Materials, 2022, 37(1): 29-37. |
| [2] | LIANG Fengqing, WEN Zhaoyin. MOF/Poly(Ethylene Oxide) Composite Polymer Electrolyte for Solid-state Lithium Battery [J]. Journal of Inorganic Materials, 2021, 36(3): 332-336. |
| [3] | WANG Yuwei, CHEN Jiajie, TIAN Zhengfang, ZHU Min, ZHU Yufang. Potassium Ferrate-loaded Porphyrin-based (VI) Metal-organic Frameworks for Combined Photodymanic and Chemodynamic Tumor Therapy [J]. Journal of Inorganic Materials, 2021, 36(12): 1305-1315. |
| [4] | ZHANG Xincong,GUO Ke,PENG Lianlian,WU Jieyu,ZHANG Fumin,ZHU Weidong,FU Yanghe. Degradation of Dye Wastewater over NH2-UiO-66: Piezoelectrically Induced Mechano-Catalytic Effect [J]. Journal of Inorganic Materials, 2020, 35(9): 1023-1028. |
| [5] | HOU Qi, WANG Maohuai, LIU Sen, DONG Hongbin, GUO Wenyue, LU Xiaoqing. Mechanisms of Hydrogen Purification in a Graphene-like Carbon Nitride Separation Membrane [J]. Journal of Inorganic Materials, 2020, 35(11): 1234-1238. |
| [6] | Shi-Qiang LUO, Chun-Man ZHENG, Wei-Wei SUN, Wei XIE, Jian-Huang KE, Shuang-Ke LIU, Xiao-Bin HONG, Yu-Jie LI, Jing XU. Controllable Preparation of Co-NC Nanoporous Carbon Derived from ZIF-67 for Advanced Lithium-sulfur Batteries [J]. Journal of Inorganic Materials, 2019, 34(5): 502-508. |
| [7] | HAN Li, ZHANG Xiao-Min, WU De-Yong. MoS2 Quantum Dots Decorated NH2-MIL-125 Heterojunction: Preparation and Visible Light Photocatalytic Performance [J]. Journal of Inorganic Materials, 2019, 34(11): 1205-1209. |
| [8] | WANG Xiang-Xue, YU Shu-Jun, WANG Xiang-Ke. Removal of Radionuclides by Metal-organic Framework-based Materials [J]. Journal of Inorganic Materials, 2019, 34(1): 17-26. |
| [9] | HUA Cheng-Jiang, WANG Ming-Hui, LUAN Guo-You, LIU Yan, WU Hua. Rapid in situ Crystallization and Catalytic Performance of Cu3(BTC)2-based Film on Copper Mesh [J]. Journal of Inorganic Materials, 2015, 30(5): 529-534. |
| [10] | GUO Yan-Rong, CHANG Wei, ZHANG Wen, WANG Hui. Photocatalytic Properties of MOF-derived ZnO/C, Ag/ZnO/C Porous Composite Materials [J]. Journal of Inorganic Materials, 2015, 30(12): 1321-1326. |
| [11] | YANG Dong-Hua, WANG Xin-Bo, SHI Bao-Bao, WU Zheng-Huang, Li Xiao-Feng, DOU Tao. Synthesis of ZSM-5/EU-1 Composite Zeolite and Its Application in Conversion of Methanol to Xylene [J]. Journal of Inorganic Materials, 2014, 29(4): 357-363. |
| [12] | DU Shu-Hui, LIU Ya-Guang, KONG Ling-Yin, ZHANG Jian, LIU Hai-Ou, ZHANG Xiong-Fu. Seeded Secondary Growth Synthesis of ZIF-8 Membranes Supported on α-Al2O3 Ceramic Tubes [J]. Journal of Inorganic Materials, 2012, 27(10): 1105-1111. |
| [13] | FU Gang,CHEN Zhi-Xiong,ZHANG Jin-Xiu. Complex Impedance Analysis for Sensitivity and Selectivity of SnO2 Gas Sensors [J]. Journal of Inorganic Materials, 2000, 15(3): 456-460. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||