
Journal of Inorganic Materials ›› 2018, Vol. 33 ›› Issue (1): 100-106.DOI: 10.15541/jim20170138
• Orginal Article • Previous Articles Next Articles
WANG Dong1,2, HE Jiao-Qiao2, LAI Xiao-Fang3,4, HUANG Rong-Tie2, SHI Ying1, HUANG Fu-Qiang2,3
Received:2017-03-27
Published:2018-01-23
Online:2017-12-15
Supported by:CLC Number:
WANG Dong, HE Jiao-Qiao, LAI Xiao-Fang, HUANG Rong-Tie, SHI Ying, HUANG Fu-Qiang. Na1.88Bi1.88S4 and Na1.36Ca1.28Bi1.36S4 Single Crystals: Growth, Structure and Optical Property[J]. Journal of Inorganic Materials, 2018, 33(1): 100-106.
| Formula | Na1.88Bi1.88S4 | Na1.36Ca1.28Bi1.36S4 |
|---|---|---|
| Formula weight/(g•mol-1) | 563.42 | 495.02 |
| Temperature/K | 273 | 273 |
| Wavelength/nm | 0.07107 | 0.07107 |
| Space group | Fm-3m | Fm-3m |
| Crystal system | cubic | cubic |
| Unit cell/nm | 0.57715(3) | 0.5745(4) |
| V/nm3 | 0.19225(3) | 0.1896(4) |
| Z | 1 | 1 |
| ρ/(g•cm-3) | 4.866 | 4.336 |
| Index ranges | -6 ≤ h ≤ 6, -6 ≤k≤ 6, -6 ≤ l ≤ 6 | -7 ≤ h ≤7, -7 ≤k≤ 7, -7≤l≤ 6 |
| Reflns collected | 1428 | 773 |
| Unique reflns | 18 | 22 |
| Goodness-of- fits on F2 | 1.447 | 1.317 |
| R1[I > 2σ(I)] | 0.0106(2) | 0.0154(2) |
| wR2[I > 2σ(I)] | 0.0236(2) | 0.0314(2) |
| R1(all) | 0.0106(2) | 0.0154(2) |
| wR2(all) | 0.0236(2) | 0.0314(2) |
Table 1 The crystal data and refinement details for Na1.88Bi1.88S4 and Na1.36Ca1.28Bi1.36S4
| Formula | Na1.88Bi1.88S4 | Na1.36Ca1.28Bi1.36S4 |
|---|---|---|
| Formula weight/(g•mol-1) | 563.42 | 495.02 |
| Temperature/K | 273 | 273 |
| Wavelength/nm | 0.07107 | 0.07107 |
| Space group | Fm-3m | Fm-3m |
| Crystal system | cubic | cubic |
| Unit cell/nm | 0.57715(3) | 0.5745(4) |
| V/nm3 | 0.19225(3) | 0.1896(4) |
| Z | 1 | 1 |
| ρ/(g•cm-3) | 4.866 | 4.336 |
| Index ranges | -6 ≤ h ≤ 6, -6 ≤k≤ 6, -6 ≤ l ≤ 6 | -7 ≤ h ≤7, -7 ≤k≤ 7, -7≤l≤ 6 |
| Reflns collected | 1428 | 773 |
| Unique reflns | 18 | 22 |
| Goodness-of- fits on F2 | 1.447 | 1.317 |
| R1[I > 2σ(I)] | 0.0106(2) | 0.0154(2) |
| wR2[I > 2σ(I)] | 0.0236(2) | 0.0314(2) |
| R1(all) | 0.0106(2) | 0.0154(2) |
| wR2(all) | 0.0236(2) | 0.0314(2) |
| Atom | Symmetry | x | y | z | | Occupancy |
|---|---|---|---|---|---|---|
| Na1.88Bi1.88S4 | ||||||
| Na | 4a | 0.5 | 1.0 | 0.5 | 0.0222(1) | 0.47(2) |
| Bi | 4a | 0.5 | 1.0 | 0.5 | 0.0222(1) | 0.47(2) |
| S | 4b | 1.0 | 0.5 | 0.5 | 0.0210(2) | 1.00 |
| Na1.36Ca1.28Bi1.36S4 | ||||||
| Na | 4b | 0.5 | 0.5 | 0.5 | 0.0600(1) | 0.34(2) |
| Ca | 4b | 0.5 | 0.5 | 0.5 | 0.0500(9) | 0.32(3) |
| Bi | 4b | 0.5 | 0.5 | 0.5 | 0.0170(3) | 0.34(2) |
| S | 4a | 1.0 | 0.5 | 0.5 | 0.0210(3) | 1.00 |
Table 2 Atomic coordinates of Na1.88Bi1.88S4 and Na1.36Ca1.28Bi1.36S4
| Atom | Symmetry | x | y | z | | Occupancy |
|---|---|---|---|---|---|---|
| Na1.88Bi1.88S4 | ||||||
| Na | 4a | 0.5 | 1.0 | 0.5 | 0.0222(1) | 0.47(2) |
| Bi | 4a | 0.5 | 1.0 | 0.5 | 0.0222(1) | 0.47(2) |
| S | 4b | 1.0 | 0.5 | 0.5 | 0.0210(2) | 1.00 |
| Na1.36Ca1.28Bi1.36S4 | ||||||
| Na | 4b | 0.5 | 0.5 | 0.5 | 0.0600(1) | 0.34(2) |
| Ca | 4b | 0.5 | 0.5 | 0.5 | 0.0500(9) | 0.32(3) |
| Bi | 4b | 0.5 | 0.5 | 0.5 | 0.0170(3) | 0.34(2) |
| S | 4a | 1.0 | 0.5 | 0.5 | 0.0210(3) | 1.00 |
| Atom | U11 | U22 | U33 | U12 | U13 | U23 |
|---|---|---|---|---|---|---|
| Na1.88Bi1.88S4 | ||||||
| Na | 0.0222(3) | 0.0222(3) | 0.0222(3) | 0 | 0 | 0 |
| Bi | 0.0222(3) | 0.0222(3) | 0.0222(3) | 0 | 0 | 0 |
| S | 0.0213(5) | 0.0213(5) | 0.0213(5) | 0 | 0 | 0 |
| Na1.36Ca1.28Bi1.36S4 | ||||||
| Na | 0.0588(2) | 0.0588(2) | 0.0588(2) | 0 | 0 | 0 |
| Ca | 0.0511(8) | 0.0511(8) | 0.0511(8) | 0 | 0 | 0 |
| Bi | 0.0171(6) | 0.0171(6) | 0.0171(6) | 0 | 0 | 0 |
| S | 0.0206(9) | 0.0206(9) | 0.0206(9) | 0 | 0 | 0 |
Table 3 Anisotropic displacement parameters (×10-6, nm2) of Na1.88Bi1.88S4 and Na1.36Ca1.28Bi1.36S4*
| Atom | U11 | U22 | U33 | U12 | U13 | U23 |
|---|---|---|---|---|---|---|
| Na1.88Bi1.88S4 | ||||||
| Na | 0.0222(3) | 0.0222(3) | 0.0222(3) | 0 | 0 | 0 |
| Bi | 0.0222(3) | 0.0222(3) | 0.0222(3) | 0 | 0 | 0 |
| S | 0.0213(5) | 0.0213(5) | 0.0213(5) | 0 | 0 | 0 |
| Na1.36Ca1.28Bi1.36S4 | ||||||
| Na | 0.0588(2) | 0.0588(2) | 0.0588(2) | 0 | 0 | 0 |
| Ca | 0.0511(8) | 0.0511(8) | 0.0511(8) | 0 | 0 | 0 |
| Bi | 0.0171(6) | 0.0171(6) | 0.0171(6) | 0 | 0 | 0 |
| S | 0.0206(9) | 0.0206(9) | 0.0206(9) | 0 | 0 | 0 |
| Atom-atom | Bond length | Atom-atom-atom | Bond angle/(°) |
|---|---|---|---|
| Na1.88Bi1.88S4 | |||
| Na(1)-S(1) | 0.28857(5) | S(1)-Na(1)-S(1) | 90 |
| Bi(1)-S(1) | 0.28857(5) | S(1)-Bi(1)-S(1) | 90 |
| Na1.36Ca1.28Bi1.36S4 | |||
| Na(1)-S(1) | 0.2872(2) | S(1)-Na(1)-S(1) | 90 |
| Ca(1)-S(1) | 0.2872(2) | S(1)-Ca(1)-S(1) | 90 |
| Bi(1)-S(1) | 0.2872(2) | S(1)-Bi(1)-S(1) | 90 |
Table 4 Representative bond lengths (nm) and bond angles (º) of Na1.88Bi1.88S4 and Na1.36Ca1.28Bi1.36S4
| Atom-atom | Bond length | Atom-atom-atom | Bond angle/(°) |
|---|---|---|---|
| Na1.88Bi1.88S4 | |||
| Na(1)-S(1) | 0.28857(5) | S(1)-Na(1)-S(1) | 90 |
| Bi(1)-S(1) | 0.28857(5) | S(1)-Bi(1)-S(1) | 90 |
| Na1.36Ca1.28Bi1.36S4 | |||
| Na(1)-S(1) | 0.2872(2) | S(1)-Na(1)-S(1) | 90 |
| Ca(1)-S(1) | 0.2872(2) | S(1)-Ca(1)-S(1) | 90 |
| Bi(1)-S(1) | 0.2872(2) | S(1)-Bi(1)-S(1) | 90 |
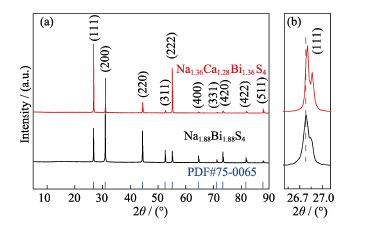
Fig. 2 (a) Powder X-ray diffraction patterns of Na1.88Bi1.88S4 (black line) and Na1.36Ca1.28Bi1.36S4 (red line), with blue bars indicating the Bragg positions for NaBiS2 phase; (b) Magnified pattern of the (111) lattice plane of Na1.88Bi1.88S4 and Na1.36Ca1.28Bi1.36S4, respectively
| [1] | CHEN JIAN-CHAI, YU CHANG-LIN, LI JIA-DE, et al.Preparation by grinding-calcination and photocatalytic performance of La2O3/BiOCl composite photocatalysts.J. Inorg. Mater., 2015, 30(9): 943-949. |
| [2] | WANG W, JIA F L, HUANG Q H, et al.Electrochemical assembled p-type Bi2Te3 thermoelectric materials with nanowire array structure.J. Inorg. Mater., 2004, 19(3): 517-522. |
| [3] | LEE J, STONE M B, HUQ A, et al.Crystal structure, lattice vibrations, and superconductivity of LaO1-xFxBiS2.Physical Review B, 2013, 87(20): 205134. |
| [4] | SHRUTI, SRIVASTAVA P, PATNAIK S.Evidence for fully gapped strong coupling s-wave superconductivity in Bi4O4S3.Journal of Physics: Condensed Matter, 2013, 25(31): 312202. |
| [5] | SHAO JIFENG, LIU ZHONGHENG, YAO XIONG, et al.Bulk superconductivity in single-phase Bi3O2S3.Physica Status Solidi (RRL) - Rapid Research Letters, 2014, 8(10): 845-848. |
| [6] | SHRUTI, MAURYA V K, NEHA P, et al. Superconductivity by Sr intercalation in the layered topological insulator.Physical Review B, 2015, 92(2): 020506. |
| [7] | BOON J W.The crystal structure of NaBiS2 and KBiS2.Recueil des Travaux Chimiques des Pays-Bas, 1944, 63(2): 32-34. |
| [8] | LAVRENT'EV A A, MIGAL' YU F, NIKIFOROV I YA. Two types of shape resonances in the compounds AIBiS2 (AI=Li, Na).Journal of Structural Chemistry, 1992, 33(2): 207-213. |
| [9] | PARK YOUNBONG, MCCARTHY TIMOTHY J,SUTORLK ANTHONY C, et al. Synthesis of Ternary Chalcogenides in Molten Polychalcogenide Salts: α-KCuQ4, KAuS5, NaBiS2, KFeQ2(Q = S, Se). Inorg. Synth., John Wiley & Sons, Inc., 2007: 88-95. |
| [10] | GABREL’YAN B V, LAVRENTIEV A A, NIKIFOROV I YA, et al. Electronic energy structure of MBiS2 (M = Li, Na, K) calculated with allowance for the difference between the M-S and Bi-S bond lengths.Journal of Structural Chemistry, 2008, 49(5): 788-794. |
| [11] | PAN LIN, B RARDAN DAVID, DRAGOE NITA. High thermoelectric properties of n-Type AgBiSe2.Journal of the American Chemical Society, 2013, 135(13): 4914-4917. |
| [12] | KANG SUMIN, HONG YONGHOON, JEON YOUNGJIN.A facile synthesis and characterization of sodium bismuth sulfide (NaBiS2) under hydrothermal condition.Bulletin of the Korean Chemical Society, 2014, 45(38): 279-287. |
| [13] | CHEN XIAOBO, CLEMENS BURDA.The electronic origin of the visible-light absorption properties of C-, N- and S-doped TiO2 nanomaterials.Journal of the American Chemical Society, 2008, 130(15): 5018-5019. |
| [14] | ABDUKADER ABDUKAYUM, AILIJIANG TUERDI, RENAGUL ABDURAHMAN, et al.Synthesis and luminescence properties of Dy,Cr co-doped ZnGa2O4 persistent luminescence nanoparticles.J. Inorg. Mater., 2016, 31(12): 1363-1369. |
| [15] | ZHANG ZHI-XIONG, OUYANG SHAO-YE,ZHANG Yue-Pin et al.. Enhanced luminescent properties of Pr3+ doped Ba2LaF7 glass ceramics for white light-emitting diodes.J. Inorg. Mater., 2016, 31(10): 1046-1050. |
| [16] | KAMIHARA YOICHI, WATANABE TAKUMI, HIRANO MASAHIRO, et al.Iron-based layered superconductor La[O1-xFx] FeAs (x = 0.05-0.12) with Tc = 26 K.Journal of the American Chemical Society, 2008, 130(11): 3296-3297. |
| [17] | NAZIA U S, ISLAM A K M A. Model spectral function and superconductivity in BaxK1-xBiO3 (x=0.5-0.7).Solid State Communications, 2003, 125(1): 37-40. |
| [18] | NGUYEN T N, GIAQUINTA D M, DAVIS W M, et al.Electrosynthesis of KBiO3: a potassium ion conductor with the KSbO3 tunnel structure.Chemistry of Materials, 1993, 5: 1273-1276. |
| [19] | SHIRAI M, SUZUKI N, MOTIZUKI K.Superconductivity in BaPb1-xBixO3 and BaxK1-xBiO3.Journal of Physics: Condensed Matter, 1989, 1(17): 2939. |
| [20] | CHEN CHENG-LUNG, WANG HENG, CHEN YANG-YUAN, et al.Thermoelectric properties of p-type polycrystalline SnSe doped with Ag.Journal of Materials Chemistry A, 2014, 2(29): 11171-11176. |
| [21] | LI L, XIAO J Y, CUI M D, et al.Boron and sulfur co-doped TiO2 nanofilm as high efficiency CdS quantum-dot-sensitized solar cells.J. Inorg. Mater., 2016, 31(6): 627-633. |
| [22] | SUN TONG, CHEN YANG, MA XIAO-QING, et al.Facile synthesis of visible light activated carbon-incorporated Mn doped TiO2 microspheres via flame thermal method.J. Inorg. Mater., 2015, 30(9): 1002-1008. |
| [23] | FRANCART TOM, VAN WIERINGEN ASTRID, WOUTERS JAN.APEX 3: a multi-purpose test platform for auditory psychophysical experiments.Journal of Neuroscience Methods, 2008, 172(2): 283-293. |
| [24] | SHELDRICK GEORGE.Crystal structure refinement with SHELXL.Acta Crystallographica Section C, 2015, 71(1): 3-8. |
| [25] | SHELDRICK G M, SADABS. Program for Empirical Absorption Correction of Area Detector Data. University of Göttingen, Germany, 1996. |
| [26] | CHRISTY ALFRED A, KVALHEIM OLAV M, VELAPOLDI RANCE A.Quantitative analysis in diffuse reflectance spectrometry: a modified Kubelka-Munk equation.Vib. Spectrosc, 1995, 9(1): 19-27. |
| [27] | SPEK A.Single-crystal structure validation with the program PLATON.J. Appl. Crystallogr., 2003, 36(1): 7-13. |
| [28] | WEST C D.The crystal structures of some alkali hydrosulfides and monosulfides.Zeitschrift für Kristallographie-Crystalline Materials, 1934, 88: 97. |
| [29] | PRIMAK W, KAUFMAN H, WARD R.X-ray diffraction studies of systems involved in the preparation of alkaline earth sulfide and selenide phosphors.Journal of the American Chemical Society, 1948, 70: 2043-2046. |
| [30] | KUPČ K V,VESEL-NOV KOV LUDMILA.Zur Kristallstruktur des Bismuthinits, Bi2S3.Tschermaks Mineralogische und Petrographische Mitteilungen, 1970, 14(1): 55-59. |
| [31] | KUDO AKIHIKO, NIISHIRO RYO, IWASE AKIHIDE, et al.Effects of doping of metal cations on morphology, activity, and visible light response of photocatalysts.Chem. Phys., 2007, 339(1/2/3): 104-110. |
| [32] | FANG L, WANG Y, ZOU P Y, et al.Fabrication and superconductivity of NaxTaS2 crystals.Physical Review B, 2005, 72(1): 014534. |
| [33] | CHRISSAFIS K, ZAMANI M, KAMBAS K, et al.Structural studies of MoS2 intercalated by lithium.Materials Science and Engineering: B, 1989, 3(1): 145-151. |
| [34] | YANG Y, GAO J, CUI J R, et al.Research progress of perovskite solar cells.J. Inorg. Mater., 2015, 30(11): 1131-1138. |
| [35] | MATSUO YUTAKA, ICHIKI TAKAHIKO, NAKAMURA EIICHI.Molecular photoelectric switch using a mixed SAM of organic [60]Fullerene and [70]Fullerene doped with a single iron atom.Journal of the American Chemical Society, 2011, 133(25): 9932-9937. |
| [36] | WANG W Q, ZHENG H F, LU G H, et al.Recent progress on applications of nano metal oxides in perovskite solar cells.J. Inorg. Mater., 2016, 31(9): 897-907. |
| [1] | FAN Xiu-Jun, WANG Yue, XU Hong. Growth and Defects Study of A:Al2O3(A=Cr, Fe, Ni) Single Crystals [J]. Journal of Inorganic Materials, 2011, 26(12): 1266-1272. |
| [2] | HE Zhi-Yu, ZHAO Bei-Jun, ZHU Shi-Fu, CHEN Bao-Jun, LI Jia-Wei, ZHANG Yi, DU Wen-Juan. Polycrystal Synthesis and Single Crystal Growth of CdGeAs2 [J]. Journal of Inorganic Materials, 2010, 25(11): 1195-1198. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||