
Journal of Inorganic Materials ›› 2018, Vol. 33 ›› Issue (1): 41-47.DOI: 10.15541/jim20170157
• Orginal Article • Previous Articles Next Articles
XU Cong-Bin1,2, YANG Wen-Jie3, SUN Hong-Liang3, LIU Wei-Jiang3, YANG Yuan-Yu1, LIN Ai-Jun1
Received:2017-04-05
Revised:2017-05-12
Published:2018-01-23
Online:2017-12-15
Supported by:CLC Number:
XU Cong-Bin, YANG Wen-Jie, SUN Hong-Liang, LIU Wei-Jiang, YANG Yuan-Yu, LIN Ai-Jun. Performance and Mechanism of Pb(II) Removal by Expanded Graphite Loaded with Zero-Valent Iron[J]. Journal of Inorganic Materials, 2018, 33(1): 41-47.
| Kinetic model | k/((mg·L)1-n·min-1) | R2 |
|---|---|---|
| First-order model | 0.0771 | 0.9885 |
| Second-order model | 0.0053 | 0.9450 |
| Third-order model | 0.0005 | 0.9340 |
Table 1 Kinetic parameters for the removal of Pb(II) by EG-ZVI
| Kinetic model | k/((mg·L)1-n·min-1) | R2 |
|---|---|---|
| First-order model | 0.0771 | 0.9885 |
| Second-order model | 0.0053 | 0.9450 |
| Third-order model | 0.0005 | 0.9340 |
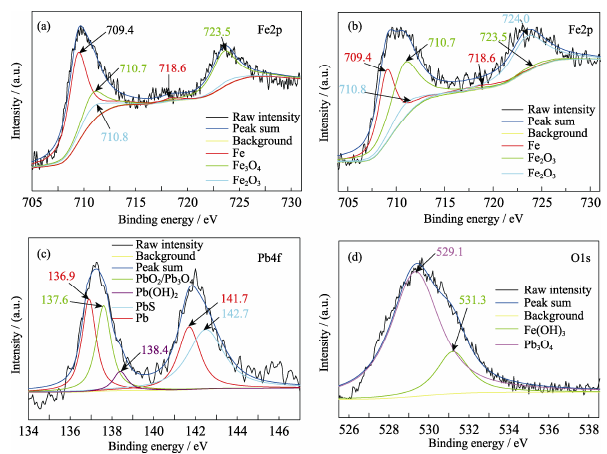
Fig. 7 XPS spectra of the EG-ZVI^(a) Fe2p before Pb(II) removal; (b) Fe2p after Pb(II) removal; (c) Pb4f after Pb(II) removal; (d) O1s after Pb(II) removal
| [1] | ChANDRAIAH M R. Facile synthesis of zero valent iron magnetic biochar composites for Pb(II) removal from the aqueous medium.Alex. Eng. J., 2016, 55(1): 619-625. |
| [2] | TAO Y G, YE L B, PAN J, et al.Removal of Pb(II) from aqueous solution on chitosan/TiO2 hybrid film.J. Hazard. Mater., 2009, 161(2/3): 718-722. |
| [3] | SELATNIA A, BOUKAZOULA A, KECHILD H N, et al.Biosorption of lead (II) from aqueous solution by a bacterial dead Streptomyces rimosus biomass.Biochem. Eng. J., 2004, 19(2): 127-135. |
| [4] | LU Y, HELUO J G.An improved synthesis of chitosan bead for Pb(II) adsorption.Chem. Eng. J., 2013, 226(24): 271-278. |
| [5] | KAPOOR A, VIRARAGHAVAN T, CULLIMORE D R.Removal of heavy metals using the fungus Aspergillus niger.Biores. Tech., 1999, 70(1): 95-104. |
| [6] | CHEN R Y, ZHANG P L, LIAO S L, et al.Electrochemical removal of low concentration Pb(II) from aqueous solution based on PPy/α-ZrP/PTCF electrode.J. Electrochem., 2015, 21(4): 344-352. |
| [7] | LUO S, LU T, PENG L, et al.Synthesis of nanoscale zero-valent iron immobilized in alginate microcapsules for removal of Pb(II) from aqueous solution. J. Mater. Chem. A, 2014, 2(37): 15463-15472. |
| [8] | FANG Y T, DING J, FAN J, et al.Preparation and performance of novel A13+modified silica geladsorptive materials.J. Inorg. Mater., 2005, 20(4): 933-939. |
| [9] | LI Y P, SUN C J, JIA K, et al.Soft template-directed hierarchical mordenites and their performance in benzylation of benzene with benzyl alcohol.J. Inorg. Mater., 2016, 31(12): 1355-1362. |
| [10] | WU M J, GAO Z Y, YUAN J, et al.Hydrothermal fabrication and catalytic performance of chromium oxide for low-concentration NO oxidation at ambient temperature.J. Inorg. Mater., 2016, 31(11): 1191-1197. |
| [11] | LIU P, WANG X, JUN M A, et al.Application of enhanced composite technologies of nano zero-valent iron in the treatment of water pollution.Chem. Ind. Eng., 2016, 35(3): 926-934. |
| [12] | JABEEN H, KEMP K C, CHANDRA V.Synthesis of nano zerovalent iron nanoparticles--graphene composite for the treatment of lead contaminated water. J. Environ. Manage., 2013, 130(1): 429-435. |
| [13] | LI Z J, WANG L, YUAN L Y, et al.Efficient removal of uranium from aqueous solution by zero-valent iron nanoparticle and its graphene composite.J. Hazard. Mater., 2015, 290: 26-33. |
| [14] | HUANG Q, LIU W, PENG P, et al.Reductive dechlorination of tetrachlorobisphenol A by Pd/Fe bimetallic catalysts.J. Hazard. Mater., 2013, 262(22): 634-641. |
| [15] | HU C Y.Hexavalent chromium removal from near natural water by copper-iron bimetallic particles.Water Res., 2010, 44(10): 3101-3108. |
| [16] | XU F, DENG S, XU J, et al.Highly active and stable Ni-Fe bimetal prepared by ball milling for catalytic hydrodechlorination of 4-chlorophenol.Environ. Sci. Technol., 2012, 46(8): 4576-4582. |
| [17] | ZhOU X, JING G, LV B, et al. Highly efficient removal of chromium(VI) by Fe/Ni bimetallic nanoparticles in an ultrasound- assisted system.Chemosphere, 2016, 160: 332-341. |
| [18] | FU F, CHENG Z, DIONYSION D D, et al.Fe/Al bimetallic particles for the fast and highly efficient removal of Cr(VI) over a wide pH range: performance and mechanism.J. Hazard. Mater., 2015, 298: 261-269. |
| [19] | SU Y F, CHENG Y L, SHIH Y H.Removal of trichloroethylene by zerovalent iron/activated carbon derived from agricultural wastes.J. Environ. Manage., 2013, 129(129C): 361-366. |
| [20] | SUN Y, DING C, CHENG W, et al.Simultaneous adsorption and reduction of U(VI) on reduced graphene oxide-supported nanoscale zerovalent iron.J. Hazard. Mater., 2014, 280: 399-408. |
| [21] | WANG H M.Effect of agitation on sorption behavior of expanded graphite for methyl orange in water and crude oil floated on water.Adv. Mater. Res., 2012, 496: 391-394. |
| [22] | YANG L, LIU H, DENG Q, et al.Modification of expandable graphite and adsorption for methyl orange.Appl. Mech. Mater., 2014, 618: 81-85. |
| [23] | ZHU M C, YANG T, HUANG J B, et al.Removal of methyl orange from aqueous solution onto expanded graphite by adsorption process.Adv. Mater. Res., 2011, 322: 89-92. |
| [24] | MENG Z, JIA Z B, WEI Y.Preparation and FTIR spectra of amorphous δ-FeOOH.J. Process Eng., 2004, 4(2): 146-149. |
| [25] | SU C, PULS R W.Kinetics of trichloroethene reduction by zerovalent iron and tin: pretreatment effect, apparent activation energy, and intermediate products.Environ. Sci. Technol., 1999, 33(1): 163-168. |
| [26] | HUA L J, YUN Z J, PING Z T.Assessment of apparent activation energies for reducing iron oxides by CO and CO-H2.J. Iron Res., 2000, 34(1): 5-9. |
| [27] | LIU J, ZHANG J, ZHOU T.Assessment of apparent activation energies for reduction reactions of iron oxides by hydrogen.J. Iron Res., 1999, 11(6): 9-13. |
| [28] | MATHIEU H J, LANDOLT D.An investigation of thin oxide films thermally grown in situ on Fe24Cr and Fe24Cr11Mo by auger electron spectroscopy and X-ray photoelectron spectroscopy.Corros. Sci., 1986, 26(7): 547-559. |
| [29] | HAWN D D, DEKOVEN B M.Deconvolution as a correction for photoelectron inelastic energy losses in the core level XPS spectra of iron oxides.Surf. Interface Anal., 1987, 10(2/3): 63-74. |
| [30] | TAN B J, SHERWOOD P M A, KLABUNDE K J.XPS studies of gold films prepared from nonaqueous gold colloids.Langmuir, 1990, 6(1): 105-113. |
| [31] | MILLS P, SULLIWAN J L.A study of the core level electrons in iron and its three oxides by means of X-ray photoelectron spectroscopy.J. Phys. D Appl. Phys., 1983, 16(5): 723-732. |
| [32] | CHAUHAN P K, GADIYAR H S, KRISHNAN R.X-ray photoelectron spectroscopy for surface film analysis in corrosion research.Pramana, 1985, 24(1): 383-395. |
| [33] | BLAKE P G, CARLEY A F, CASTRO V D, et al.Chemisorptive replacement of surface oxygen by hydrogen halides (HCl and HBr) at Pb(110) surfaces. Photoelectron spectroscopic and kinetic evidence for a metastable chloride overlayer.J. Chem. Soc., Faraday Trans., 1986, 82(3): 723-737. |
| [34] | NEFEDOV V I, SALYN Y V, SOLOZHENKIN P M, et al.X-ray photoelectron study of surface compounds formed during flotation of minerals.Surf. Interface Anal., 1980, 2(5): 170-172. |
| [35] | ROGERS J D, SUNDARAM V S, KLEIMAN G G, et al.High resolution study of the M45N67N67 and M45N45N67 Auger transitions in the 5d series.J. Phys. F Metal Phys., 1982, 12(9): 2097-2102. |
| [36] | BARR T L, YIN M, VARMA S.Detailed X-ray photoelectron spectroscopy valence band and core level studies of select metals oxidations. J. Vac. Sci. Technol., 1992, 10(4): 2383-2390. |
| [37] | TAYLOR J A, PERRY D L.An X-ray photoelectron and electron energy loss study of the oxidation of lead.J. Vac. Sci. Technol., 1984, 2(2): 771-774. |
| [38] | ETTEMA A R H F, HAAS C. An X-ray photoemission spectroscopy study of interlayer charge transfer in some misfit layer compounds.J. Phys. Condens. Matter, 1993, 5(23): 3817-3826. |
| [39] | LIU H, JIANG E Y, ZHENG R K, et al.Structures and transport properties of polycrystalline Fe3O4 filmsJ. Phys. Condens. Matter, 2003, 15(15): 8003-8009. |
| [1] | LI Jing,LIU Xiaoyue,QIU Qianfeng,LI Ling,CAO Xiaoyan. Phosphorus Sorption Characteristics on Aluminum Oxides with Different Structures [J]. Journal of Inorganic Materials, 2020, 35(9): 1005-1010. |
| [2] | ZHAO Chaofeng, JIN Jiaren, HUO Yingzhong, SUN Lu, AI Yuejie. Adsorption of Phenolic Organic Pollutants on Graphene Oxide: Molecular Dynamics Study [J]. Journal of Inorganic Materials, 2020, 35(3): 277-283. |
| [3] | LI Jian, ZHANG Gang-Hua, FAN Li-Kun, HUANG Guo-Quan, GAO Zhi-Peng, ZENG Tao. Enhanced Visible-light-driven Photocatalytic Activity of Multiferroic KBiFe2O5 by Adjusting pH Value [J]. Journal of Inorganic Materials, 2018, 33(7): 805-810. |
| [4] | YU Jie-Yi, HUANG Hao, GAO Jian, ZHOU Lei, GAO Song, DONG Xing-Long, QUAN Xie. Synthesis and Catalytic Performances of SiC Nanoparticles by DC Arc-discharge Plasma [J]. Journal of Inorganic Materials, 2017, 32(4): 351-356. |
| [5] | WEI Jian-Wen, WANG Ji-Hua, ZHAO Song-Sheng, GENG Lin-Lin. Synthesis and Copper(II) Affinity Performance of Amidoxime Functionalized Mesoporous Silica [J]. Journal of Inorganic Materials, 2016, 31(5): 449-453. |
| [6] | ZENG Tao, BAI Yang, LI Hao, MAO Chao-Liang, DONG Xian-Lin, GUI Shu-Xiang. Fabrication of Barium Strontium Titanate Nanophotocatalysts with Gridding Structures and Their Photocatalytic Activities [J]. Journal of Inorganic Materials, 2015, 30(12): 1334-1338. |
| [7] |
ZENG Tao, BAI Yang, LI Hao, YAO Wei-Feng, DONG Xian-Lin.
Preparation of Polyhedral Copper Oxide Nanoparticles by Molten-salt Method and Their Catalytic Performance [J]. Journal of Inorganic Materials, 2015, 30(4): 439-442. |
| [8] |
CHEN Yi,SHI Li-Yi,YUAN Shuai,WU Jun,ZHANG Mei-Hong,FANG Jian-Hui.
Photoelectrocatalytic Degradation of Methylene Blue by TiO2 Nanotube Array Prepared by Anodic Oxidation [J]. Journal of Inorganic Materials, 2009, 24(4): 680-684. |
| [9] | ZHANG Jun,WANG De-Ping,YAO Ai-Hua,HUANG Wen-Hai. Research on Adsorption Capacity for Ni2+ and Mechanism of Nano-hydroxyapatite [J]. Journal of Inorganic Materials, 2009, 24(2): 269-274. |
| [10] | QIAN Guang-Ren,BAI Hong-Mei,SUN Fu-Cheng,ZHOU Ji-Zhi,SUN Wei-Min,XU Xia. Preparation and Stability of Calcium Cadmium Hydroxyapatite [J]. Journal of Inorganic Materials, 2008, 23(5): 1016-1020. |
| [11] | SUN Hai-Jian,LIU Hui-Ling. Preparation of Cerium-doped TiO2/Ti Photoelectrodes and Photoelectrocatalytic Performance under Visible Light [J]. Journal of Inorganic Materials, 2007, 22(6): 1065-1069. |
| [12] | LI Xiao-Zhong,YE Qun-Feng,WANG Lian-Jun. Polyaluminum Chloride with High Al13 Content Rapidly Prepared by Electrolysis with Exchanging Electrodes [J]. Journal of Inorganic Materials, 2007, 22(6): 1211-1215. |
| [13] |
CAO Jiang-Lin,WU Zu-Cheng,CAO Fa-He,ZHANG Jian-Qing.
Cathodic Co-electrodeposition of Fe3+-doped TiO2 Thin Films and Their Photocatalytic Activity under Visible Light [J]. Journal of Inorganic Materials, 2007, 22(3): 514-518. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||