
Journal of Inorganic Materials ›› 2017, Vol. 32 ›› Issue (11): 1165-1170.DOI: 10.15541/jim20170023
• RESEARCH PAPER • Previous Articles Next Articles
CHAI Er-Ya1, PAN Jun-An1, YUAN Guo-Long1, CHENG Hao1, AN Feng1, XIE Shu-Hong2
Received:2017-01-11
Revised:2017-02-28
Published:2017-11-20
Online:2017-10-20
CLC Number:
CHAI Er-Ya, PAN Jun-An, YUAN Guo-Long, CHENG Hao, AN Feng, XIE Shu-Hong. Preparation and Electrochemical Property of Polyaniline Coated Opal Shale/Sulfur Composite[J]. Journal of Inorganic Materials, 2017, 32(11): 1165-1170.
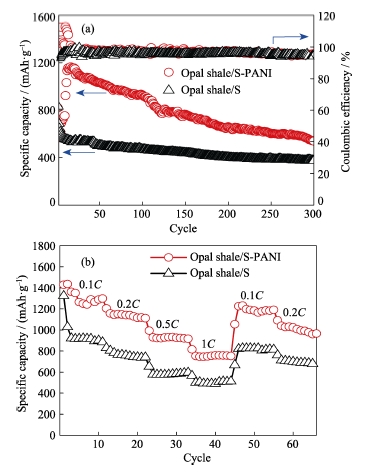
Fig. 5 (a) Cycling performance and the corresponding coulombic efficiency at 0.5C rate; (b) rate capability under different discharge rate of the opal shale/S and opal shale/S- PANI
| [1] | HU J J, LI G R, GAO X P.Current status, problems and challenges in lithium-sulfur batteries.Journal of Inorganic Materials, 2013, 28(11): 1181-1186. |
| [2] | YANG Y, ZHENG G Y, CUI Y.Nanostructured sulfur cathodes.Chemical Society Reviews, 2013, 42(7): 3018-3032. |
| [3] | BRUCE P G, FREUNBERGER S A, HARDWICK L J,et al. Li-O2 and Li-S batteries with high energy storage. Nature Materials, 2011, 11(1): 19-29. |
| [4] | JI X L, NAZAR L F.Advances in Li-S batteries.Journal of Materials Chemistry, 2010, 20(44): 9821-9826. |
| [5] | ZHENG J M, GU M, WANG C M,et al. Controlled nucleation and growth process of Li2S2/Li2S in lithium-sulfur batteries. Journal of the Electrochemical Society, 2013, 160(11): A1992-A1996. |
| [6] | SAH Z W, LI W Y, CHA J J,et al. Sulphur-TiO2 yolk-shell nanoarchitecture with internal void space for long-cycle lithium- sulphur batteries. Nature Communications, 2013, 4: 1331-1336. |
| [7] | MIKHAYLIK Y V, AKRIDGE J R.Polysulfide shuttle study in the Li/S battery system.Journal of the Electrochemical Society, 2004, 151(11): A1969-A1976. |
| [8] | JI X L, EVERS S, BLACK R,et al. Stabilizing lithium-sulphur cathodes using polysulphide reservoirs. Nature Communications, 2011, 2: 325-331. |
| [9] | KIM M, KANG S H, MANUEL J,et al. Investigation into the role of silica in lithium polysulfide adsorption for lithium sulfur battery. Materials Research Bulletin, 2015, 69: 29-35. |
| [10] | ZHAO X F, HE C Y, LI X L.A novel adsorbent material opal shale.North Environment, 2005, 30(2): 62-63. |
| [11] | YANG D F, WEI C D, NING W K,et al. Structure and adsorption properties of nenjiang opal shale. Journal of Jilin University(Earth Science Edition), 2010, 40(5): 1061-1065. |
| [12] | LI X L, ZHAO X F, ZHANG J L.Study on decolorization of new adsorption material opal shale.North Environment, 2004, 29(1): 37-38. |
| [13] | KIM J H, SEO J, CHOI J,et al. Synergistic ultrathin functional polymer-coated carbon nanotube interlayer for high performance lithium-sulfur batteries. ACS Applied Materials & Interfaces, 2016, 8(31): 20092-20099. |
| [14] | SUN Q, HE B, ZHANG X Q,et al. Engineering of hollow core-shell interlinked carbon spheres for highly stable lithium- sulfur batteries. ACS Nano, 2015, 9(8): 8504-8513. |
| [15] | WU F, CHEN J Z, CHEN R J,et al. Sulfur/polythiophene with a core/shell structure: synthesis and electrochemical properties of the cathode for rechargeable lithium batteries. The Journal of Physical Chemistry C, 2011, 115(13): 6057-6063. |
| [16] | LI G C, LI G R, YE S H,et al. A polyaniline-coated sulfur/carbon composite with an enhanced high-rate capability as a cathode material for lithium/sulfur batteries. Advanced Energy Materials, 2012, 2(10): 1238-1245. |
| [17] | ZHOU W D, YU Y C, CHEN H,et al. Yolk-shell structure of polyaniline-coated sulfur for lithium-sulfur batteries. Journal of the American Chemical Society, 2013, 135(44): 16736-16743. |
| [18] | GUSTAFSSON G, CAO Y, TREACY G M,et al. Flexible light- emitting diodes made from soluble conducting polymers. Nature, 1992, 357(6378): 477-479. |
| [19] | KRUK M, JARONIEC M.Gas adsorption characterization of ordered organic-inorganic nanocomposite materials.Chemistry of Materials, 2001, 13(10): 3169-3183. |
| [20] | WANG Z D, ZHANG M L, MEI H Y,et al. The physical chemistry explanation of the capillary condensation and the circuit of adsorption-desorption. Xinjiang Petroleum Geology, 2002, 23(3): 233-235. |
| [21] | LI W Y, ZHENG G Y, YANG Y,et al. High-performance hollow sulfur nanostructured battery cathode through a scalable, room temperature, one-step, bottom-up approach. Proceedings of the National Academy of Sciences, 2013, 110(18): 7148-7153. |
| [22] | ATEBAMBA J M, MOSKON J, PEJOVNIK S,et al. On the interpretation of measured impedance spectra of insertion cathodes for lithium-ion batteries. Journal of the Electrochemical Society, 2010, 157(11): A1218-A1228. |
| [23] | CHEN J J, JIA X, SHE Q J,et al. The preparation of nano- sulfur/MWCNTs and its electrochemical performance. Electrochimica Acta, 2010, 55(27): 8062-8066. |
| [1] | KONG Guoqiang, LENG Mingzhe, ZHOU Zhanrong, XIA Chi, SHEN Xiaofang. Sb Doped O3 Type Na0.9Ni0.5Mn0.3Ti0.2O2 Cathode Material for Na-ion Battery [J]. Journal of Inorganic Materials, 2023, 38(6): 656-662. |
| [2] | YANG Zhuo, LU Yong, ZHAO Qing, CHEN Jun. X-ray Diffraction Rietveld Refinement and Its Application in Cathode Materials for Lithium-ion Batteries [J]. Journal of Inorganic Materials, 2023, 38(6): 589-605. |
| [3] | LI Tao, CAO Pengfei, HU Litao, XIA Yong, CHEN Yi, LIU Yuejun, SUN Aokui. NH4+ Assisted Interlayer-expansion of MoS2: Preparation and Its Zinc Storage Performance [J]. Journal of Inorganic Materials, 2023, 38(1): 79-86. |
| [4] | WANG Yang, FAN Guangxin, LIU Pei, YIN Jinpei, LIU Baozhong, ZHU Linjian, LUO Chengguo. Microscopic Mechanism of K+ Doping on Performance of Lithium Manganese Cathode for Li-ion Battery [J]. Journal of Inorganic Materials, 2022, 37(9): 1023-1029. |
| [5] | LI Gaoran, LI Hongyang, ZENG Haibo. Recent Progress of Boron-based Materials in Lithium-sulfur Battery [J]. Journal of Inorganic Materials, 2022, 37(2): 152-162. |
| [6] | LI Wenbo, HUANG Minsong, LI Yueming, LI Chilin. CoS2 as Cathode Material for Magnesium Batteries with Dual-salt Electrolytes [J]. Journal of Inorganic Materials, 2022, 37(2): 173-181. |
| [7] | LI Tingting, ZHANG Yang, CHEN Jiahang, MIN Yulin, WANG Jiulin. Flexible Binder for S@pPAN Cathode of Lithium Sulfur Battery [J]. Journal of Inorganic Materials, 2022, 37(2): 182-188. |
| [8] | JIANG Hao,WU Hao,HOU Chengyi,LI Yaogang,XIAO Ru,ZHANG Qinghong,WANG Hongzhi. Sawing Angles on Property of Lithium-sulfur Battery Interlayer Prepared with Birch Derived Orientedly Microchannel Biochar [J]. Journal of Inorganic Materials, 2020, 35(6): 717-723. |
| [9] | ZHANG Wei,GAO Peng,HOU Chengyi,LI Yaogang,ZHANG Qinghong,WANG Hongzhi. Chip Sensor for pH and Temperature Monitoring Based on ZnO Composite [J]. Journal of Inorganic Materials, 2020, 35(4): 416-422. |
| [10] | Ya-Dong LI, Wei-Ping LI, Qin WANG, Dao-Guang ZHENG, Jian-Xin WANG. Flexible Carbon-fiber Supported Carbon-sulfur Electrode: Preparation, Physical Property and Electrochemical Performance [J]. Journal of Inorganic Materials, 2019, 34(4): 373-378. |
| [11] | HU Xi, LIU Hong-Bo, XIA Xiao-Hong, GU Zhi-Qiang. Polyaniline-carbon Pillared Graphene Composite: Preparation and Electrochemical Performance [J]. Journal of Inorganic Materials, 2019, 34(2): 145-151. |
| [12] | WANG Wu-Lian, ZHANG Jun, WANG Qiu-Shi, CHEN Liang, LIU Zhao-Ping. High-quality Fe4[Fe(CN)6]3 Nanocubes: Synthesis and Electrochemical Performance as Cathode Material for Aqueous Sodium-ion Battery [J]. Journal of Inorganic Materials, 2019, 34(12): 1301-1308. |
| [13] | WANG Jia-Hu, WANG Wen-Xin, DU Peng, HU Fang-Dong, JIANG Xiao-Lei, YANG Jian. Synthesis of Na3V2(PO4)2F3@V2O5-x as Cathode Material for Sodium-ion Battery [J]. Journal of Inorganic Materials, 2019, 34(10): 1097-1102. |
| [14] | LEE Sai-Xi, WANG Xue-Yin, GU Qing-Wen, XIA Yong-Gao, LIU Zhao-Ping, HE Jie. Tuning Electrochemical Performance through Non-stoichiometric Compositions in High-voltage Spinel Cathode Materials [J]. Journal of Inorganic Materials, 2018, 33(9): 993-1000. |
| [15] | LUO Ling-Hong, HU Jia-Xing, CHENG Liang, XU Xu, WU Ye-Fan, LIN You-Chen. Performance of the Composite Cathode Ba0.5Sr0.5Co0.8Fe0.2O3-δ-Ce0.9Gd0.1O2-δ for Medium-low Temperature Solid Oxide Fuel Cell [J]. Journal of Inorganic Materials, 2018, 33(4): 441-446. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||