
Journal of Inorganic Materials ›› 2017, Vol. 32 ›› Issue (5): 459-468.DOI: 10.15541/jim20160452
• Orginal Article • Previous Articles Next Articles
TIAN Xiao-Dong1, 2, LI Xiao1, 2, YANG Tao1, 2, SONG Yan1, LIU Zhan-Jun1, GUO Quan-Gui1
Received:2016-08-08
Revised:2016-09-19
Published:2017-05-20
Online:2017-05-02
About author:TIAN Xiao-Dong. E-mail: tianxiaodong0124@163.com
CLC Number:
TIAN Xiao-Dong, LI Xiao, YANG Tao, SONG Yan, LIU Zhan-Jun, GUO Quan-Gui. Recent Advances on Synthesis and Supercapacitor Application of Binary Metal Oxide[J]. Journal of Inorganic Materials, 2017, 32(5): 459-468.
| Materials | Parameters | Specific capacitance | Ref. |
|---|---|---|---|
| NiMoO4 nanospheres | H2O, 140℃, 12 h | 974.4 F/g (1 A/g) | [27] |
| NiMoO4 nanorods | Ethanol-H2O, 140℃, 12 h | 944.8 F/g (1 A/g) | |
| MnMoO4 nanosheets on Ni foam | H2O, 150℃, 8 h | 1271 F/g (5 mV/s) | [28] |
| CoMoO4 nanoplate arrays on Ni foam | H2O, 180℃, 12 h | 1.26 F/cm2 (4 mA/cm2) | [29] |
| NiMoO4 nanowire arrays on carbon cloth | H2O, 140℃, 8 h | 414.7 F/g (0.25 A/g) | [30] |
Table 1 Parameters and performance of BTMOs synthesized by solvothermal method
| Materials | Parameters | Specific capacitance | Ref. |
|---|---|---|---|
| NiMoO4 nanospheres | H2O, 140℃, 12 h | 974.4 F/g (1 A/g) | [27] |
| NiMoO4 nanorods | Ethanol-H2O, 140℃, 12 h | 944.8 F/g (1 A/g) | |
| MnMoO4 nanosheets on Ni foam | H2O, 150℃, 8 h | 1271 F/g (5 mV/s) | [28] |
| CoMoO4 nanoplate arrays on Ni foam | H2O, 180℃, 12 h | 1.26 F/cm2 (4 mA/cm2) | [29] |
| NiMoO4 nanowire arrays on carbon cloth | H2O, 140℃, 8 h | 414.7 F/g (0.25 A/g) | [30] |
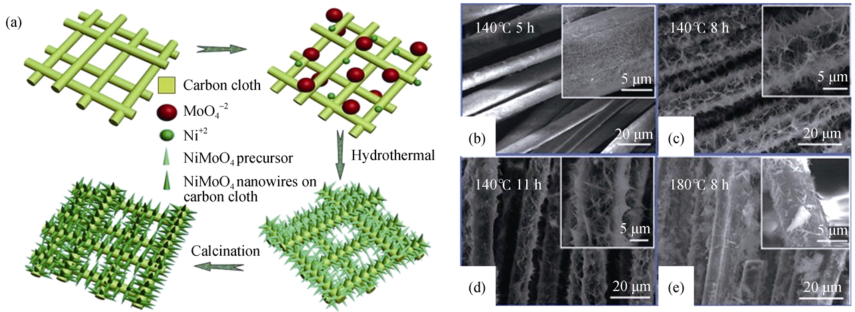
Fig. 1 (a) Schematic illustration of the formation processes of the NiMoO4 NW arrays on carbon cloth via hydrothermal; SEM images of the prepared NiMoO4 NW arrays on carbon cloth at 140℃ for different hours (b-d) and SEM inage of NiMoO4 NW arrays at 180℃(e) [30]
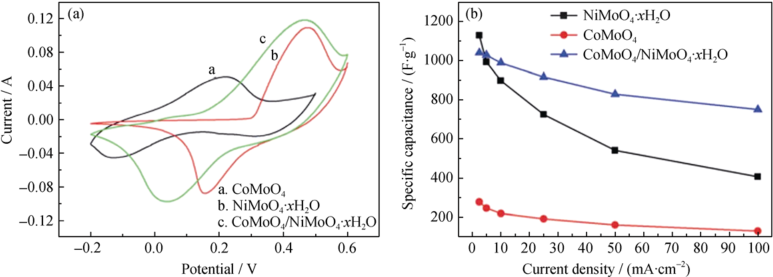
Fig. 4 (a) CV curves of NiMoO4·xH2O, CoMoO4, and CoMoO4-NiMoO4·xH2O bundles at a scan rate of 20 mV/s; (b) Specific capacitances of NiMoO4·xH2O, CoMoO4, and CoMoO4-NiMoO4·xH2O bundles at controlled current densities[42]
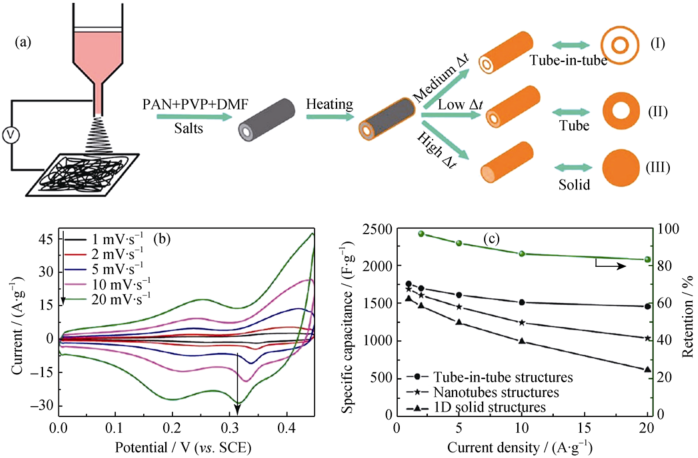
Fig. 5 (a) Schematic illustration for the formation process of the ternary TMOs with complex 1D nanostructures including tube-in-tube, nanotube, and solid 1D nanostructures; (b) CV curves of NiCo2O4 tube-in-tube nanostructures at various scan rates ranging from 1-20 mV•s-1; (c) the specific capacitance at various current density of NiCo2O4 tube-in-tube, nanotube, and 1D solid nanostructures[47]
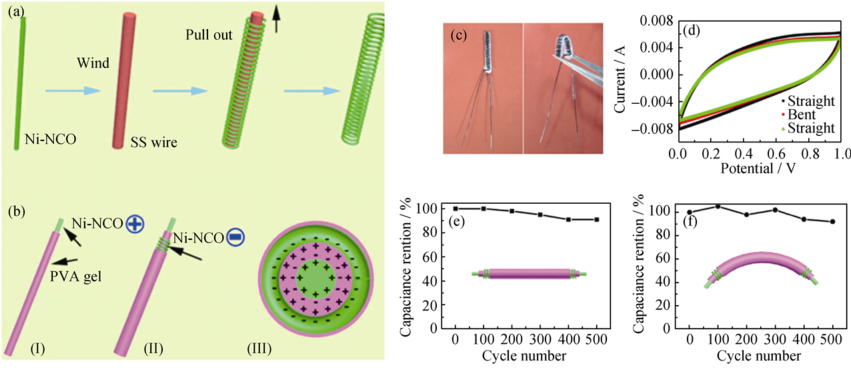
Fig. 7 Schematic diagram showing (a) the fabrication of the winding electrode and (b) the fabrication process of the fiber solid-state NCO SCs; (c) Photos of fiber SCs in straight and bending states; (d) CV curves at 80 mV/s in straight and bending states, respectively. (e, f) The capacitance stability of fiber SCs in straight and bending states for 500 cycles, respectively[48]
| Materials | Method | C/(F•g-1) | Rate capability | Cycle performance | Ref. |
|---|---|---|---|---|---|
| NiCo2O4 nanorobs on carbon nanofibers | Coprecipitation | 1026 (1 A/g) | 48.7% (20/1) | 91.5% (2 A/g, 2000) | [26] |
| NiCo2O4 nanosheets on carbon nanofibers | 1002 (1 A/g) | 51.9% (20/1) | 96.4% (2 A/g, 2400) | ||
| NiCo2O4 microspheres | Microwave assisted synthesis | 774 (2 mV/s) | 52.3% (100/2) | - | [32] |
| Ordered mesoporous NiCo2O4 | Hard template | 739 (2.86 A/g) | 55.2% (28.6/2.86) | 95%(28.6 A/g, 2500) | [38] |
| NiCo2O4 hollow submicropheres | Soft template | 987 (1 A/g) | 62.8% (50/1) | No capacity loss (5 A/g, 5000) | [40] |
| NiCo2O4 nanowires | Microemulsion | 1481 (0.5 A/g) | 42.2% (8/0.5) | 91.4% (2 A/g, 2000) | [44] |
| NiCo2O4 tube-in-tube structure | Electrospinning | 1756 (1 A/g) | 83.0% (20/1) | 92.4% (5 A/g, 5000) | [47] |
| Flower like NiCo2O4/3D graphene foam | Electrodeposition | 1402 (1 A/g) | 77.0% (20/1) | 76.6% (5 A/g, 5000) | [48] |
| NiCo2O4@Ppy nanowire arrays on carbon textiles | Hydrothermal | 2244 (1 A/g) | 60.5% (30/1) | 82.9% (3 A/g, 10000) | [54] |
| Si/NiCo2O4 heterostructure | Hydrothermal | 1972 (2 A/g) | 54.4% (10/2) | 78% (10 A/g, 2000) | [55] |
Table 2 Comparison of the electrochemical performance of NiCo2O4 obtained by different methods
| Materials | Method | C/(F•g-1) | Rate capability | Cycle performance | Ref. |
|---|---|---|---|---|---|
| NiCo2O4 nanorobs on carbon nanofibers | Coprecipitation | 1026 (1 A/g) | 48.7% (20/1) | 91.5% (2 A/g, 2000) | [26] |
| NiCo2O4 nanosheets on carbon nanofibers | 1002 (1 A/g) | 51.9% (20/1) | 96.4% (2 A/g, 2400) | ||
| NiCo2O4 microspheres | Microwave assisted synthesis | 774 (2 mV/s) | 52.3% (100/2) | - | [32] |
| Ordered mesoporous NiCo2O4 | Hard template | 739 (2.86 A/g) | 55.2% (28.6/2.86) | 95%(28.6 A/g, 2500) | [38] |
| NiCo2O4 hollow submicropheres | Soft template | 987 (1 A/g) | 62.8% (50/1) | No capacity loss (5 A/g, 5000) | [40] |
| NiCo2O4 nanowires | Microemulsion | 1481 (0.5 A/g) | 42.2% (8/0.5) | 91.4% (2 A/g, 2000) | [44] |
| NiCo2O4 tube-in-tube structure | Electrospinning | 1756 (1 A/g) | 83.0% (20/1) | 92.4% (5 A/g, 5000) | [47] |
| Flower like NiCo2O4/3D graphene foam | Electrodeposition | 1402 (1 A/g) | 77.0% (20/1) | 76.6% (5 A/g, 5000) | [48] |
| NiCo2O4@Ppy nanowire arrays on carbon textiles | Hydrothermal | 2244 (1 A/g) | 60.5% (30/1) | 82.9% (3 A/g, 10000) | [54] |
| Si/NiCo2O4 heterostructure | Hydrothermal | 1972 (2 A/g) | 54.4% (10/2) | 78% (10 A/g, 2000) | [55] |
| [1] | MANTHIRAM A, MURUGAN A V, SARKAR A, et al.Nanostructured electrode materials for electrochemical energy storage and conversion.Energy Environ. Sci., 2008, 1(6): 621-638. |
| [2] | YAN J, WANG Q, WEI T, et al.Recent advances in design and fabrication of electrochemical supercapacitors with high energy densities.Adv. Energy Mater., 2014, DOI: 10.1002/aenm.201300816. |
| [3] | WANG Y, XIA Y.Recent progress in supercapacitors: from materials design to system construction.Adv. Mater., 2013, 25(37): 5336-5342. |
| [4] | GONZ LEZ A, GOIKOLEA E, BARRENA J A, et al.Review on supercapacitors: Technologies and materials.Renewable and Sustainable Energy Reviews, 2016, 58: 1189-1206. |
| [5] | WANG Q, YAN J, FAN Z.Carbon materials for high volumetric performance supercapacitors: design, progress, challenges and opportunities.Energy Environ. Sci., 2016, 9(3): 729-762. |
| [6] | ZHAI Y, DOU Y, ZHAO D, et al.Carbon materials for chemical capacitive energy storage.Adv. Mater., 2011, 23(42): 4828-4850. |
| [7] | DENG X, ZHAO B, ZHU L, et al.Molten salt synthesis of nitrogen- doped carbon with hierarchical pore structures for use as high-performance electrodes in supercapacitors.Carbon, 2015, 93: 48-58. |
| [8] | WANG K, WU H, MENG Y, et al.Conducting polymer nanowire arrays for high performance supercapacitors.Small, 2014, 10(1): 14-31. |
| [9] | WANG Q, O’HARE D. Recent advances in the synthesis and application of layered double hydroxide (LDH) nanosheets.Chem. Rev., 2012, 112(7): 4124-4155. |
| [10] | YU M, ZENG Y, HAN Y, et al.Valence-optimized vanadium oxide supercapacitor electrodes exhibit ultrahigh capacitance and super- long cyclic durability of 100 000 cycles.Adv Funct Mater, 2015, 25(23): 3534-3540. |
| [11] | RUI X, TAN H, YAN Q.Nanostructured metal sulfides for energy storage.Nanoscale, 2014, 6(17): 9889-9924. |
| [12] | BALOGUN M S, QIU W, WANG W, et al.Recent advances in metal nitrides as high-performance electrode materials for energy storage devices.J. Mater. Chem. A, 2015, 3(4): 1364-1387. |
| [13] | SUN M, LIU H, QU J, et al.Earth-rich transition metal phosphide for energy conversion and storage.Adv. Energy Mater., 2016, DOI: 10.1002/aenm.201600087. |
| [14] | ZHI M, XIANG C, LI J, et al.Nanostructured carbon-metal oxide composite electrodes for supercapacitors: a review.Nanoscale, 2013, 5(1): 72-88. |
| [15] | DUBAL D P, GOMEZ-ROMERO P, SANKAPAL B R, et al.Nickel cobaltite as an emerging material for supercapacitors: An overview.Nano Energy, 2015, 11: 377-399. |
| [16] | SHOWN I, GANGULY A, CHEN L C, et al.Conducting polymer-based flexible supercapacitor.Energ. Sci. Eng., 2014, 3(1): 2-26. |
| [17] | WANG G, ZHANG L, ZHANG J.A review of electrode materials for electrochemical supercapacitors.Chem. Soc. Rev., 2012, 41(2): 797-828. |
| [18] | KIM S Y, JEONG H M, KWON J H, et al.Nickel oxide encapsulated nitrogen-rich carbon hollow spheres with multiporosity for high-performance pseudocapacitors having extremely robust cycle life.Energy Environ. Sci., 2015, 8(1): 188-194. |
| [19] | ABOUALI S, AKBARI GARAKANI M, ZHANG B, et al.Electrospun carbon nanofibers with in situ encapsulated Co3O4 nanoparticles as electrodes for high-performance supercapacitors.ACS Appl. Mater. Interfaces, 2015, 7(24): 13503-13511. |
| [20] | SARAVANAKUMAR B, PURUSHOTHAMAN K K, MURALIDHARAN G.Interconnected V2O5 nanoporous network for high-performance supercapacitors.ACS Appl. Mater. Interfaces, 2012, 4(9): 4484-4490. |
| [21] | GHOSH A, RA E J, JIN M, et al.High pseudocapacitance from ultrathin V2O5 films electrodeposited on self-standing carbon- nanofiber paper.Adv. Funct. Mater., 2011, 21(13): 2541-2547. |
| [22] | MENDOZA-S NCHEZ B, GRANT P S. Charge storage properties of a α-MoO3/carboxyl-functionalized single-walled carbon nanotube composite electrode in a Li ion electrolyte.Electrochim Acta, 2013, 98: 294-302. |
| [23] | FAN Z, YAN J, WEI T, et al.Asymmetric supercapacitors based on graphene/MnO2 and activated carbon nanofiber electrodes with high power and energy density.Adv. Funct. Mater., 2011, 21(12): 2366-2375. |
| [24] | CHAUDHARI N K, CHAUDHARI S, YU J S.Cube-like α-Fe2O3 supported on ordered multimodal porous carbon as high performance electrode material for supercapacitors.ChemSusChem, 2014, 7(11): 3102-3111. |
| [25] | PENG S, LI L, WU H B, et al.Controlled growth of nimoo4 nanosheet and nanorod arrays on various conductive substrates as advanced electrodes for asymmetric supercapacitors.Adv. Energy Mater., 2015, DOI: 10.1002/aenm.201401172. |
| [26] | ZHANG G, LOU X W.Controlled growth of NiCo2O4 nanorods and ultrathin nanosheets on carbon nanofibers for high-performance supercapacitors.Sci. Rep., 2013, 3: 1470. |
| [27] | CAI D, WANG D, LIU B, et al.Comparison of the electrochemical performance of NiMoO4 nanorods and hierarchical nanospheres for supercapacitor applications.ACS Appl. Mater. Interfaces, 2013, 5(24): 12905-12910. |
| [28] | MU X, ZHANG Y, WANG H, et al.A high energy density asymmetric supercapacitor from ultrathin manganese molybdate nanosheets.Electrochim Acta, 2016, 211: 217-224. |
| [29] | GUO D, ZHANG H, YU X, et al.Facile synthesis and excellent electrochemical properties of CoMoO4 nanoplate arrays as supercapacitors.J. Mater. Chem. A, 2013, 1(24): 7247-7254. |
| [30] | WANG C, XI Y, HU C, et al.β-NiMoO4 nanowire arrays grown on carbon cloth for 3D solid asymmetry supercapacitor.RSC Adv., 2015, 5: 107098-107104. |
| [31] | WAN H, JIANG J, JI X, et al.Rapid microwave-assisted synthesis NiMoO4·H2O nanoclusters for supercapacitors.Mater. Lett., 2013, 108: 164-167. |
| [32] | KHALID S, CAO C, WANG L, et al.Microwave assisted synthesis of porous NiCo2O4 microspheres: application as high performance asymmetric and symmetric supercapacitors with large areal capacitance.Sci. Rep., 2016, 6: 22699. |
| [33] | DU J, ZHOU G, ZHANG H, et al.Ultrathin porous NiCo2O4 nanosheet arrays on flexible carbon fabric for high-performance supercapacitors.ACS Appl. Mater. Interfaces, 2013, 5(15): 7405-7409. |
| [34] | WU J, GUO P, MI R, et al.Ultrathin NiCo2O4 nanosheets grown on three-dimensional interwoven nitrogen-doped carbon nanotubes as binder-free electrodes for high-performance supercapacitors.J. Mater. Chem. A, 2015, 3(29): 15331-15338. |
| [35] | XIAO K, XIA L, LIU G, et al.Honeycomb-like NiMoO4 ultrathin nanosheet arrays for high-performance electrochemical energy storage.J. Mater. Chem. A, 2015, 3(11): 6128-6135. |
| [36] | KONG L B, LU C, LIU M C, et al.Effect of surfactant on the morphology and capacitive performance of porous NiCo2O4.J. Solid State Electrochem., 2013, 17(5): 1463-1471. |
| [37] | HSU C T, HU C C.Synthesis and characterization of mesoporous spinel NiCo2O4 using surfactant-assembled dispersion for asymmetric supercapacitors.J. Power Sources, 2013, 242: 662-671. |
| [38] | LU Q, CHEN Y, LI W, et al.Ordered mesoporous nickel cobaltite spinel with ultra-high supercapacitance.J. Mater. Chem. A, 2013, 1(6): 2331-2336. |
| [39] | YUAN C, LI J, HOU L, et al.Template-engaged synthesis of uniform mesoporous hollow NiCo2O4 sub-microspheres towards high-performance electrochemical capacitors.RSC Adv., 2013, 3(40): 18573-18578. |
| [40] | ZHU Y, WANG J, WU Z, et al.An electrochemical exploration of hollow NiCo2O4 submicrospheres and its capacitive performances.J. Power Sources, 2015, 287: 307-315. |
| [41] | LIU M C, KANG L, KONG L B, et al.Facile synthesis of NiMoO4·xH2O nanorods as a positive electrode material for supercapacitors.RSC Adv., 2013, 3(18): 6472-6478. |
| [42] | LIU M C, KONG L B, LU C, et al.Design and synthesis of CoMoO4-NiMoO4·xH2O bundles with improved electrochemical properties for supercapacitors.J. Mater. Chem. A, 2013, 1(4): 1380-1387. |
| [43] | HAETGE J, DJERDJ I, BREZESINSKI T.Nanocrystalline NiMoO4 with an ordered mesoporous morphology as potential material for rechargeable thin film lithium batteries.Chem. Commun., 2012, 48(53): 6726-6728. |
| [44] | AN C, WANG Y, HUANG Y, et al.Porous NiCo2O4 nanostructures for high performance supercapacitors via a microemulsion technique.Nano Energy, 2014, 10: 125-134. |
| [45] | LONG C, ZHENG M, XIAO Y, et al.Amorphous Ni-Co binary oxide with hierarchical porous structure for electrochemical capacitors.ACS Appl. Mater. Interfaces, 2015, 7(44): 24419-24429. |
| [46] | CAI D, LIU B, WANG D, et al.Facile hydrothermal synthesis of hierarchical ultrathin mesoporous NiMoO4 nanosheets for high performance supercapacitors.Electrochim. Acta, 2014, 115: 358-363. |
| [47] | PENG S, LI L, HU Y, et al.Fabrication of spinel one-dimensional architectures by single-spinneret electrospinning for energy storage applications.ACS Nano, 2015, 9(2): 1945-1954. |
| [48] | ZHANG C, KUILA T, KIM N H, et al.Facile preparation of flower- like NiCo2O4/three dimensional graphene foam hybrid for high performance supercapacitor electrodes.Carbon, 2015, 89: 328-339. |
| [49] | ZHOU J, HUANG Y, CAO X, et al.Two-dimensional NiCo2O4 nanosheet-coated three-dimensional graphene networks for high-rate, long-cycle-life supercapacitors.Nanoscale, 2015, 7(16): 7035-7039. |
| [50] | WANG Q, WANG X, XU J, et al.Flexible coaxial-type fiber supercapacitor based on NiCo2O4 nanosheets electrodes.Nano Energy, 2014, 8: 44-51. |
| [51] | WANG X, YAN C, SUMBOJA A, et al.High performance porous nickel cobalt oxide nanowires for asymmetric supercapacitor.Nano Energy, 2014, 3: 119-126. |
| [52] | AI Y, GENG X, LOU Z, et al.Rational synthesis of branched CoMoO4@CoNiO2 core/shell nanowire arrays for all-solid-state supercapacitors with improved performance.ACS Appl. Mater. Interfaces, 2015, 7(43): 24204-24211. |
| [53] | CAI D, WANG D, LIU B, et al.Three-dimensional Co3O4@NiMoO4 core/shell nanowire arrays on Ni foam for electrochemical energy storage.ACS Appl Mater Interfaces, 2014, 6(7): 5050-5055. |
| [54] | KONG D, REN W, CHENG C, et al.Three-dimensional NiCo2O4@polypyrrole coaxial nanowire arrays on carbon textiles for high-performance flexible asymmetric solid-state supercapacitor.ACS Appl Mater Interfaces, 2015, 7(38): 21334-21346. |
| [55] | MA M, GUO J, ZHANG Y, et al.Si/NiCo2O4 heterostructures electrodes with enhanced performance for supercapacitor.RSC Adv., 2015, 5(77): 62813-62818. |
| [1] | DING Ling, JIANG Rui, TANG Zilong, YANG Yunqiong. MXene: Nanoengineering and Application as Electrode Materials for Supercapacitors [J]. Journal of Inorganic Materials, 2023, 38(6): 619-633. |
| [2] | WANG Bo, YU Jian, LI Cuncheng, NIE Xiaolei, ZHU Wanting, WEI Ping, ZHAO Wenyu, ZHANG Qingjie. Service Stability of Gd/Bi0.5Sb1.5Te3 Thermo-electro-magnetic Gradient Composites [J]. Journal of Inorganic Materials, 2023, 38(6): 663-670. |
| [3] | YANG Zhuo, LU Yong, ZHAO Qing, CHEN Jun. X-ray Diffraction Rietveld Refinement and Its Application in Cathode Materials for Lithium-ion Batteries [J]. Journal of Inorganic Materials, 2023, 38(6): 589-605. |
| [4] | CHEN Qiang, BAI Shuxin, YE Yicong. Highly Thermal Conductive Silicon Carbide Ceramics Matrix Composites for Thermal Management: a Review [J]. Journal of Inorganic Materials, 2023, 38(6): 634-646. |
| [5] | LIN Junliang, WANG Zhanjie. Research Progress on Ferroelectric Superlattices [J]. Journal of Inorganic Materials, 2023, 38(6): 606-618. |
| [6] | NIU Jiaxue, SUN Si, LIU Pengfei, ZHANG Xiaodong, MU Xiaoyu. Copper-based Nanozymes: Properties and Applications in Biomedicine [J]. Journal of Inorganic Materials, 2023, 38(5): 489-502. |
| [7] | MA Xiaosen, ZHANG Lichen, LIU Yanchao, WANG Quanhua, ZHENG Jiajun, LI Ruifeng. 13X@SiO2: Synthesis and Toluene Adsorption [J]. Journal of Inorganic Materials, 2023, 38(5): 537-543. |
| [8] | ZHANG Shuo, FU Qiangang, ZHANG Pei, FEI Jie, LI Wei. Influence of High Temperature Treatment of C/C Porous Preform on Friction and Wear Behavior of C/C-SiC Composites [J]. Journal of Inorganic Materials, 2023, 38(5): 561-568. |
| [9] | YUAN Jingkun, XIONG Shufeng, CHEN Zhangwei. Research Trends and Challenges of Additive Manufacturing of Polymer-derived Ceramics [J]. Journal of Inorganic Materials, 2023, 38(5): 477-488. |
| [10] | CHEN Xinli, LI Yan, WANG Weisheng, SHI Zhiwen, ZHU Liqiang. Gelatin/Carboxylated Chitosan Gated Oxide Neuromorphic Transistor [J]. Journal of Inorganic Materials, 2023, 38(4): 421-428. |
| [11] | DU Jianyu, GE Chen. Recent Progress in Optoelectronic Artificial Synapse Devices [J]. Journal of Inorganic Materials, 2023, 38(4): 378-386. |
| [12] | YANG Yang, CUI Hangyuan, ZHU Ying, WAN Changjin, WAN Qing. Research Progress of Flexible Neuromorphic Transistors [J]. Journal of Inorganic Materials, 2023, 38(4): 367-377. |
| [13] | YOU Junqi, LI Ce, YANG Dongliang, SUN Linfeng. Double Dielectric Layer Metal-oxide Memristor: Design and Applications [J]. Journal of Inorganic Materials, 2023, 38(4): 387-398. |
| [14] | QI Zhanguo, LIU Lei, WANG Shouzhi, WANG Guogong, YU Jiaoxian, WANG Zhongxin, DUAN Xiulan, XU Xiangang, ZHANG Lei. Progress in GaN Single Crystals: HVPE Growth and Doping [J]. Journal of Inorganic Materials, 2023, 38(3): 243-255. |
| [15] | ZHANG Chaoyi, TANG Huili, LI Xianke, WANG Qingguo, LUO Ping, WU Feng, ZHANG Chenbo, XUE Yanyan, XU Jun, HAN Jianfeng, LU Zhanwen. Research Progress of ScAlMgO4 Crystal: a Novel GaN and ZnO Substrate [J]. Journal of Inorganic Materials, 2023, 38(3): 228-242. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||