
Journal of Inorganic Materials ›› 2017, Vol. 32 ›› Issue (4): 379-385.DOI: 10.15541/jim20160330
• Orginal Article • Previous Articles Next Articles
XU Hong-Mei1, 2, ZHANG Hua3, LI Heng3, JIAN Yao-Yong3, XIE Wu3, WANG Yi-Ping3, XU Ming-Ze3
Received:2016-05-19
Revised:2016-08-03
Published:2017-04-20
Online:2017-03-24
Supported by:CLC Number:
XU Hong-Mei, ZHANG Hua, LI Heng, JIAN Yao-Yong, XIE Wu, WANG Yi-Ping, XU Ming-Ze. Preparation and Oxygen-reduction Mechanism Investigation of Nanostructure LSCF-SDC Composite Cathodes[J]. Journal of Inorganic Materials, 2017, 32(4): 379-385.
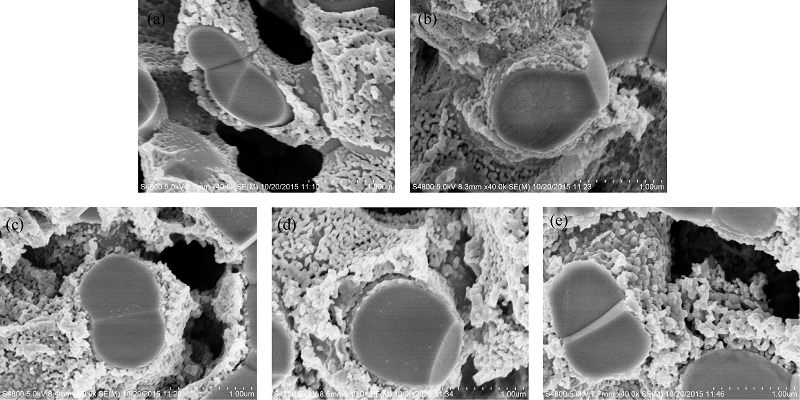
Fig. 3 SEM images of LSCF-SDC composite cathodes prepared by infiltration different LSCF loadings(a) 8.5vol%; (b) 12.3vol%; (c) 16.5vol%; (d) 20.9vol%; (e) 23.2vol%
| Loadings /vol% | n | |||
|---|---|---|---|---|
| RH | RM | RL | Rp | |
| 8.5 | 0.06 | 0.22 | 0.52 | 0.2524 |
| 12.3 | 0.01 | 0.25 | 0.55 | 0.2630 |
| 16.5 | 0.01 | 0.27 | 0.57 | 0.2900 |
| 20.9 | 0.02 | 0.26 | 0.58 | 0.2903 |
| 23.2 | 0.01 | 0.21 | 0.49 | 0.2482 |
Table 1 Reaction orders “n” of cathodes infiltrated with different LSCF loadings
| Loadings /vol% | n | |||
|---|---|---|---|---|
| RH | RM | RL | Rp | |
| 8.5 | 0.06 | 0.22 | 0.52 | 0.2524 |
| 12.3 | 0.01 | 0.25 | 0.55 | 0.2630 |
| 16.5 | 0.01 | 0.27 | 0.57 | 0.2900 |
| 20.9 | 0.02 | 0.26 | 0.58 | 0.2903 |
| 23.2 | 0.01 | 0.21 | 0.49 | 0.2482 |
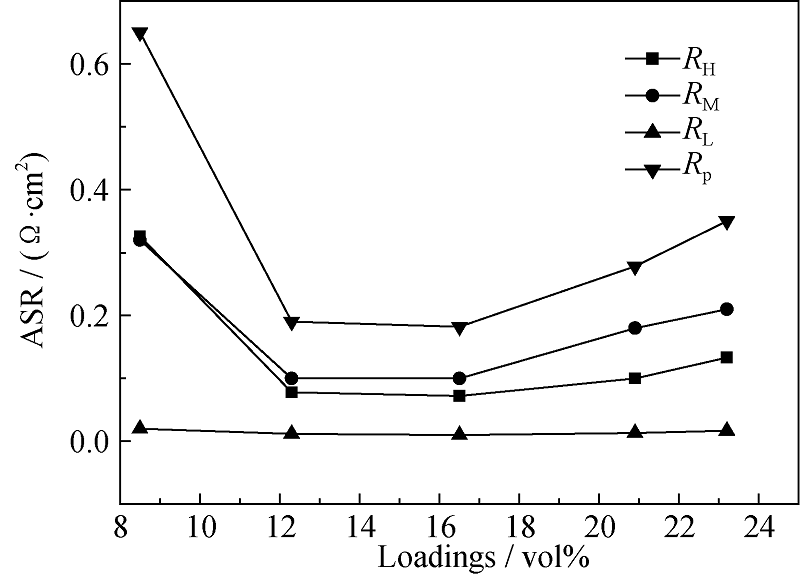
Fig. 8 LSCF loadings dependence of total cathode polarization resistance (Rp), high-frequency polarization resistance (RH), medium-frequency polarization resistance (RM) and low-frequency polarization resistance (RL) in the impedance spectra of LSCF- SDC composite cathodes
| [1] | STEEL B C H. Appraisal of Ce1-yGdyO2/y/2 electrolytes for IT-SOFC operation at 500℃. Solid State Ionics, 2000, 129(1-4): 95-110. |
| [2] | ZHEN Y D, TOK A I Y, JIANG S P, et al. Fabiraction and performance of gadolinia-doped ceria-based intermediated-temperature solid oxide fuel. Journal of Power Sources, 2008, 178(1): 69-74. |
| [3] | ADLER S B.Mechanism and kinetics reduction on porous La1-xSrxCoO3-δ electrodes. Solid State Ionics, 1998, 111(1/2): 125-134. |
| [4] | TARANCÓN A, PEÑA-MARTÍNEZ J, MARRERO-LÓPEZ D, et al. Stability, chemical compatibility and electrochemical of GdBaCo2O5+x layered perovskite as cathode for intermediate temperature solid oxide fuel cells. Solid State Ionics, 2008, 179(40): 2372-2378. |
| [5] | DING HAN-PING, XUE XING-JIAN.Layered perovskite GdBaCoFeO5+x cathode for intermediate-temperature solid oxide fuel cells. International Journal of Hydrogen Energy, 2010, 35(9): 4316-4319. |
| [6] | ZHOU WEI, RAN RAN, SHAO ZONG-PING.Progress in understanding and development of Ba0.5Sr0.5Co0.8Fe0.2O3-δ based cathodes for intermediate-temperature solid-oxide fuel cells: a review. Journal of Power Sources, 2009, 192(2): 231-246. |
| [7] | ZHANG YONG-JUN, YU BO, LU SHI-QUAN, et al.Effect of Cu doping on YBaCo2O5+δ as cathode for intermediate-temperature solid oxide fuel cells. Electrochimica Acta, 2014, 134: 107-115. |
| [8] | XIA CHANG-RONG, RAUCH WILLIAM, CHEN FANG-LIN, et al.Sm0.5Sr0.5O3 for low-temperature SOFCs. Solid State Ionics, 2002, 149(1/2): 11-19. |
| [9] | JIANG S P, WANG W.Novel structured mixed ionic and electronic conducting cathodes of solid oxide fuel cells. Solid State Ionics, 2005, 176(15/16): 1351-1357. |
| [10] | ZHAO FEI, ZHANG LEI, JIANG ZHI-YI, et al.A high performance intermediate-temperature solid oxide fuel cell using impregnated La0.6Sr0.4Co0.2Fe0.8O3-δ cathode. Journal of Alloys and Compounds, 2009, 487(1-2): 781-785. |
| [11] | HAN DA, WU HAO, LI JUN-LIANG, et al.Nanostructuring of SmBa0.5Sr0.5Co2O5+δ cathodes for reduced-temperature solid oxide fuel cells. Journal of Power Sources, 2014, 246(15): 409-416. |
| [12] | ZHOU YU-CUN, XIN XIAN-SHUANG, LI JUN-LIANG, et al.Performance and degradation of metal-supported solid oxide fuel cells with impreganted electrodes. Imternational Journal of Hydrgen Energy, 2014, 39(5): 2279-2285. |
| [13] | WANG YAO, ZHANG HAN, CHEN FANG-LIN, et al.Electrochemical characteristics of nano-structured PrBaCo2O5+x cathodes fabricated with ion impregnation process. Journal of Power Sources, 2012, 203(1): 34-41. |
| [14] | KLEMENSØ TRINE, CHATZICHRISTODOULOU CHRISTODOULOS, NIELSEN JIMMI, et al.Characterization of impregnated GDC nano-structures and their functionality in LSM based cathodes. Solid State Ionics, 2012, 224: 21-31. |
| [15] | WANG YAO, ZHANG LEI, XIA CHANG-RONG.Enhanceing oxyen surface exhange coefficients of strontium-doped lanthanum manganates with electrolytes. International Journal of Hydrogen Energy, 2012, 37(3): 2182-2186. |
| [16] | BIDRAWN F, KIM G, ARAMRUEANG N, et al.Dopants to enhance SOFC cathodes based on Sr-doped LaFeO3 and LaMnO3. Journal of Power Sources, 2010, 195(3): 720-728. |
| [17] | BUYUKAKSOY ALIGUL, PETROVSKY VLADIMIR, DOGAN FATIH.Solid oxide fuel cells with symmetrical Pt-YSZ electrodes prepared by YSZ infiltration. Journal of Electrochemical Society, 2013, 160(4): F482-F486. |
| [18] | XU HONG-MEI, YAN HONG-GE, CHEN ZHEN-HUA.Sintering and electrical properties of Ce0.8Y0.2O1.9 powders prepared by citric acid-nitrate low temperature combustion process. Journal of Power Sources, 2006, 163(1): 409-414. |
| [19] | LEE SUNG-IL, KIM JEONGHEE, SON JI-WON, et al.High performance air electrode for solid oxide regenerative fuel cells fabricated by infiltration of nano-catalysts. Journal of Power Sources, 2014, 250: 15-20. |
| [20] | SHA XUE-QING, LU ZHE, HUANG XI-QIANG, et al.Preparation and properties of rare earth co-doped Ce0.8Sm0.2-xYxO1.9 electrolyte materials for SOFC. Journal of Alloys and Compounds, 2006, 424(1-2): 315-321. |
| [21] | BAEK SEUNG-WOOK, BAE JOONGMYEON, YOO YOUNG-SUNG.Cathode reaction mechanism of porous- structured Sm0.5Sr0.5CoO3-δ and Sm0.5Sr0.5CoO3-δ/Sm0.2Ce0.8O1.9 for oxide fuel cells., Journal of Power Sources, 2009, 193(2): 431-440. |
| [1] | KONG Guoqiang, LENG Mingzhe, ZHOU Zhanrong, XIA Chi, SHEN Xiaofang. Sb Doped O3 Type Na0.9Ni0.5Mn0.3Ti0.2O2 Cathode Material for Na-ion Battery [J]. Journal of Inorganic Materials, 2023, 38(6): 656-662. |
| [2] | GUO Tianmin, DONG Jiangbo, CHEN Zhengpeng, RAO Mumin, LI Mingfei, LI Tian, LING Yihan. Enhanced Compatibility and Activity of High-entropy Double Perovskite Cathode Material for IT-SOFC [J]. Journal of Inorganic Materials, 2023, 38(6): 693-700. |
| [3] | YANG Zhuo, LU Yong, ZHAO Qing, CHEN Jun. X-ray Diffraction Rietveld Refinement and Its Application in Cathode Materials for Lithium-ion Batteries [J]. Journal of Inorganic Materials, 2023, 38(6): 589-605. |
| [4] | LI Tao, CAO Pengfei, HU Litao, XIA Yong, CHEN Yi, LIU Yuejun, SUN Aokui. NH4+ Assisted Interlayer-expansion of MoS2: Preparation and Its Zinc Storage Performance [J]. Journal of Inorganic Materials, 2023, 38(1): 79-86. |
| [5] | ZHU Hezhen, WANG Xuanpeng, HAN Kang, YANG Chen, WAN Ruizhe, WU Liming, MAI Liqiang. Enhanced Lithium Storage Stability Mechanism of Ultra-high Nickel LiNi0.91Co0.06Al0.03O2@Ca3(PO4)2 Cathode Materials [J]. Journal of Inorganic Materials, 2022, 37(9): 1030-1036. |
| [6] | WANG Yang, FAN Guangxin, LIU Pei, YIN Jinpei, LIU Baozhong, ZHU Linjian, LUO Chengguo. Microscopic Mechanism of K+ Doping on Performance of Lithium Manganese Cathode for Li-ion Battery [J]. Journal of Inorganic Materials, 2022, 37(9): 1023-1029. |
| [7] | CHEN Ying, LUAN Weiling, CHEN Haofeng, ZHU Xuanchen. Multi-scale Failure Behavior of Cathode in Lithium-ion Batteries Based on Stress Field [J]. Journal of Inorganic Materials, 2022, 37(8): 918-924. |
| [8] | SUN Lian, GU Quanchao, YANG Yaping, WANG Honglei, YU Jinshan, ZHOU Xingui. Two-dimensional Transition Metal Dichalcogenides for Electrocatalytic Oxygen Reduction Reaction [J]. Journal of Inorganic Materials, 2022, 37(7): 697-709. |
| [9] | XIA Qian, SUN Shihao, ZHAO Yiliang, ZHANG Cuiping, RU Hongqiang, WANG Wei, YUE Xinyan. Effect of Boron Carbide Particle Size Distribution on the Microstructure and Properties of Reaction Bonded Boron Carbide Ceramic Composites by Silicon Infiltration [J]. Journal of Inorganic Materials, 2022, 37(6): 636-642. |
| [10] | JIANG Lili, XU Shuaishuai, XIA Baokai, CHEN Sheng, ZHU Junwu. Defect Engineering of Graphene Hybrid Catalysts for Oxygen Reduction Reactions [J]. Journal of Inorganic Materials, 2022, 37(2): 215-222. |
| [11] | LI Wenbo, HUANG Minsong, LI Yueming, LI Chilin. CoS2 as Cathode Material for Magnesium Batteries with Dual-salt Electrolytes [J]. Journal of Inorganic Materials, 2022, 37(2): 173-181. |
| [12] | LI Tingting, ZHANG Yang, CHEN Jiahang, MIN Yulin, WANG Jiulin. Flexible Binder for S@pPAN Cathode of Lithium Sulfur Battery [J]. Journal of Inorganic Materials, 2022, 37(2): 182-188. |
| [13] | FAN Shuai, JIN Tian, ZHANG Shanlin, LUO Xiaotao, LI Chengxin, LI Changjiu. Effect of Li2O Sintering Aid on Sintering Characteristics and Electrical Conductivity of LSGM Electrolyte for Solid Oxide Fuel Cell [J]. Journal of Inorganic Materials, 2022, 37(10): 1087-1092. |
| [14] | LIU Ziruo, LIU Wei, HAO Ce, HU Jinwen, SHI Yantao. Honeycomb-like Carbon-supported Fe Single Atom Catalyst: Preparation and Electrocatalytic Performance in Oxygen Reduction Reaction [J]. Journal of Inorganic Materials, 2021, 36(9): 943-949. |
| [15] | HAO Ce, LIU Ziruo, LIU Wei, SHI Yantao. Research Progress of Carbon-supported Metal Single Atom Catalysts for Oxygen Reduction Reaction [J]. Journal of Inorganic Materials, 2021, 36(8): 820-834. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||