
Journal of Inorganic Materials ›› 2016, Vol. 31 ›› Issue (10): 1073-1080.DOI: 10.15541/jim20160173
• Orginal Article • Previous Articles Next Articles
YANG Guang-Wu1,2, YANG Rui-Xia1,3, ZHANG Shou-Chao2, ZHU Fei2
Received:2016-03-23
Revised:2016-05-18
Published:2016-10-20
Online:2016-09-23
About author:YANG Guang-Wu. E-mail: yanggw204@163.com
Supported by:CLC Number:
YANG Guang-Wu, YANG Rui-Xia, ZHANG Shou-Chao, ZHU Fei. Ce Doping Concentration on Luminescence Property of YVO4:Ce3+ Crystals[J]. Journal of Inorganic Materials, 2016, 31(10): 1073-1080.
| Samples | Cell parameter/nm | Cell Volume/nm3 V=a2c | |
|---|---|---|---|
| a=b | c | ||
| YVO4 | 0.712140 | 0.629113 | 0.319050 |
| 1.0at% | 0.712641 | 0.630389 | 0.320148 |
| 2.0at% | 0.712730 | 0.630577 | 0.320323 |
| 3.0at% | 0.712782 | 0.630741 | 0.320453 |
| 5.0at% | 0.712823 | 0.63081 | 0.320525 |
| 8.0at% | 0.713023 | 0.630851 | 0.320725 |
| 10.0at% | 0.713103 | 0.631013 | 0.320880 |
Table 1 Cell parameters of YVO4:Ce3+ compound
| Samples | Cell parameter/nm | Cell Volume/nm3 V=a2c | |
|---|---|---|---|
| a=b | c | ||
| YVO4 | 0.712140 | 0.629113 | 0.319050 |
| 1.0at% | 0.712641 | 0.630389 | 0.320148 |
| 2.0at% | 0.712730 | 0.630577 | 0.320323 |
| 3.0at% | 0.712782 | 0.630741 | 0.320453 |
| 5.0at% | 0.712823 | 0.63081 | 0.320525 |
| 8.0at% | 0.713023 | 0.630851 | 0.320725 |
| 10.0at% | 0.713103 | 0.631013 | 0.320880 |
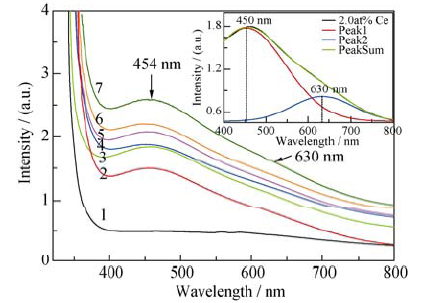
Fig. 3 Absorption spectra and Gauss fitting of YVO4 and YVO4: Ce3+ crystals (line 1 to 7 represents absorption spectrum of YVO4 and 1.0at%, 2.0at%, 3.0at%, 5.0at%, 8.0at% and 10.0at% YVO4:Ce3+ crystals, respectively)
| Ce Concentration | Ce3+ Binding energy/eV | Ce4+ Binding energy/eV | Ce3+ Relative content/% | Ce4+ Relative content/% | Ce3+ Rela content/% | Ce4+ Rela content/% |
|---|---|---|---|---|---|---|
| 1.0at% | 891.1,906.8 | 916.3 | 64.8 | 35.2 | 0.613% | 0.333% |
| 2.0at% | 884.2,904.3 | 898.1,916.2 | 75.0 | 25.0 | 1.426% | 0.476% |
| 3.0at% | 886.3,904.8 | 898.1,898.3,915.6 | 65.4 | 34.6 | 1.896% | 1.003% |
| 5.0at% | 885.4,904.6 | 882.5,899.3,916.7 | 69.0 | 31.0 | 3.284% | 1.45% |
| 8.0at% | 885.3,903.8 | 882.7,898.5,914.6 | 68.2 | 31.8 | 5.356% | 2.498% |
| 10.0at% | 884.5,903.1 | 882.6,899.2,915.7 | 71.4 | 28.6 | 6.877% | 2.755% |
Table 2 Ce3+/Ce4+ relative content in YVO4:Ce3+ crystals
| Ce Concentration | Ce3+ Binding energy/eV | Ce4+ Binding energy/eV | Ce3+ Relative content/% | Ce4+ Relative content/% | Ce3+ Rela content/% | Ce4+ Rela content/% |
|---|---|---|---|---|---|---|
| 1.0at% | 891.1,906.8 | 916.3 | 64.8 | 35.2 | 0.613% | 0.333% |
| 2.0at% | 884.2,904.3 | 898.1,916.2 | 75.0 | 25.0 | 1.426% | 0.476% |
| 3.0at% | 886.3,904.8 | 898.1,898.3,915.6 | 65.4 | 34.6 | 1.896% | 1.003% |
| 5.0at% | 885.4,904.6 | 882.5,899.3,916.7 | 69.0 | 31.0 | 3.284% | 1.45% |
| 8.0at% | 885.3,903.8 | 882.7,898.5,914.6 | 68.2 | 31.8 | 5.356% | 2.498% |
| 10.0at% | 884.5,903.1 | 882.6,899.2,915.7 | 71.4 | 28.6 | 6.877% | 2.755% |
| Ce concentration | V4+ binding energy/eV | V5+ binding energy/eV | V4+ relative content/% | V5+ relative content/% |
|---|---|---|---|---|
| 1.0at% | 515.5 | 517 | 0.24 | 99.76 |
| 2.0at% | 515.5 | 517 | 0.32 | 99.68 |
| 3.0at% | 515.5 | 517 | 0.58 | 99.42 |
| 5.0at% | 515.5 | 517 | 0.8 | 99.2 |
| 8.0at% | 515.5 | 517 | 1.37 | 98.63 |
| 10.0at% | 515.5 | 517 | 1.62 | 98.38 |
Table 3 V4+/V5+ relative content in YVO4:Ce3+ crystals
| Ce concentration | V4+ binding energy/eV | V5+ binding energy/eV | V4+ relative content/% | V5+ relative content/% |
|---|---|---|---|---|
| 1.0at% | 515.5 | 517 | 0.24 | 99.76 |
| 2.0at% | 515.5 | 517 | 0.32 | 99.68 |
| 3.0at% | 515.5 | 517 | 0.58 | 99.42 |
| 5.0at% | 515.5 | 517 | 0.8 | 99.2 |
| 8.0at% | 515.5 | 517 | 1.37 | 98.63 |
| 10.0at% | 515.5 | 517 | 1.62 | 98.38 |
| [1] | LI P L, WANG Y, GUO Q L.Research progress in single host white light emitting phosphor for white LEDs.Chinese Sci. Bull., 2011, 56(7): 488-503. |
| [2] | LI P L, WANG Z D, LUO Z Y, et al.Progress of phosphors for UV and near-UV based white LEDs. Journal of Synthetic Crystals, 2015, 44(11): 2954-2962. |
| [3] | ZHANG S H, ZHOU M B, HU J F, et al.Research progress in preparation of single phase silicate phosphor for nuv-white light emitting diodes.Materials Review, 2009, 23(5): 25-29. |
| [4] | WANG Z J, TIAN Z, YOU J Q, et al.Recent development on single-phase white emitting phosphors for white LEDs.Journal of the Chinese Ceramic Society, 2016, 44(1): 172-180. |
| [5] | RAUT S K, DHOBLE N S, DHOBLE S J.Optical properties of Eu, Dy, Mn activated M2SiO4,(M2=Ca, Sr, Zn) orthosilicate phosphors.Journal of Luminescence, 2013, 134: 325-332. |
| [6] | P S THAKER, S C GEDAM, S J DHOBLE, et al.Luminescence of KCaSO4Cl: X, Y (X=Eu or Ce; Y=Dy or Mn) halosulfate material.Journal of Luminescence, 2011, 131: 1612-1616. |
| [7] | RIWOTZKI K, HAASE M.Wet-chemical synthesis of doped colloidal nanoparticles: YVO4: Ln (Ln=Eu, Sm, Dy). The Journal of Physical Chemistry B, 1998, 102(50): 10129-10135. |
| [8] | SHEN L J, LI B, WANG Z Z, et al.Research development of rare earth vanadate liuminesence materials.Chinese Rare Earhts, 2015, 36(6): 129-137. |
| [9] | WITOLD R R, TOMASZ N, JAROS L K.Luminescence and energy transfer phenomena in YVO4 single crystal co-doped with Tm3+ and Eu3+.Journal of Luminescence, 2015, 162: 134-139. |
| [10] | BLASSE G.On the Eu3+ fluorescence of mixed metal oxides. IV. The photoluminescent efficiency of Eu3+-activated oxides.The Journal of Chemical Physics, 1966, 45(7): 2356-2360. |
| [11] | SHEN L J, LI B, WANG Z Z, et al.Vacuum ultraviolet spectra of YVO4:Tm3+.Chinese Journal of Luminescence, 2014, 35(9): 1034-1039. |
| [12] | DEVI C V, SINGH N R.Effect of annealing on the luminescence properties of YVO4: Dy phosphor on co-doping Pb2+ ions.Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 2015, 146: 331-341. |
| [13] | CHEN X B, ZHOU G, ZHOU Y F, et al.Near-Infrared quantum cutting downconversion luminescence of Yb3+ ion cooperative energy transferred from YVO4 matrix donor. Spectroscopy and Spectral Analysis, 2015, 35(2): 315-319. |
| [14] | ZHANG S C, GU J X, JIA G Z, et al.Effect of annealing on the spectroscopy performance of YVO4: Ce3+ single crystal.Optical Materials, 2015, 39: 178-181. |
| [15] | ZHANG S C, RUAN R F, JIA G Z, et al.Blue-emitting properties of Ce3+ doped YVO4 under ultraviolet excitation.Journal of Inorganic Materials, 2014, 29(10): 1067-1072. |
| [16] | PAN Y X, WANG W, LIU G K, et al.Correlation between structure variation and luminescence red shift in YAG:Ce.Journal of Alloys and Compounds, 2009, 488: 638-642. |
| [17] | XIN J, YU Y C, LIU J J, et al.Synthesis of fluorescent YVO4: Eu nano-particle and its application in developing fingerprint.Chemical Research, 2010, 21(2): 1-6. |
| [18] | WANG Y F, WANG S, RUAN Y F, et al.Photoluminescence properties of Ce and Eu co-doped YVO4 crystals.Journal of Alloys and Compounds, 2013, 551: 262-266. |
| [19] | REN G H, PEI Y, WU Y T, et al. Influence of Ce doping concentration on the luminescence properties of LaCl3:Ce scintillation crystals. Acta Physica Sinica, 2014, 63(3): 037802-1-6. |
| [20] | WANG D J.Theoretical Calculation of Electronic Structures and 4f→5d Transitions of Lanthanide Ions Doped in Crystals. Anhui: University of Science and Technology of China A Dissertation for Doctor's Degree, 2009. |
| [21] | NINGTHOUJAM R S, SINGH L R, SUDARSAN V, et al.Energy transfer process and optimum emission studies in luminescence of core-shell nanoparticles: YVO4:Eu-YVO4 and surface state analysis.Journal of Alloys and Compounds, 2009, 484: 782-789. |
| [22] | P DORENBOS.5d-level energies of Ce3+ and the crystalline environment. IV. Aluminates and “simple” oxides.Journal of Luminescence, 2002, 99: 283-299. |
| [23] | DENG T G, XIA Z G, DING H.Effect of [PO4]3-/[VO4]3- substitution on the structure and luminescence properties of Ca5[(P,V)O4]]3F: Eu3+ phosphors.Chemical Physics Letters, 2015, 637: 67-70. |
| [24] | BLASSE G, A BRIL. Luminescence of phosphors based on host lattices ABO4 (A is Sc, In; B is P, V, Nb).The Journal of Chemical Physics, 1969, 50(7): 2974-2980. |
| [25] | SVITASHEVA S N, GILINSK A M.Influence of doping level on shift of the absorption edge of gallium nitride films (Burstein-Moss effect).Applied Surface Science, 2013, 281: 109-112. |
| [26] | DORENBOS P.The 5d level positions of the trivalent lanthanides in inorganic compounds.Journal of Luminescence, 2000, 91: 155-176. |
| [27] | ANANDAN C, BERA P.XPS studies on the interaction of CeO2 with silicon in magnetronsputtered CeO2 thin films on Si and Si3N4 substrates.Applied Surface Science, 2013, 283: 297-303. |
| [28] | HEIKKINEN H, JOHANSSON L S, NYKANEN E, et al.An XPS study of SrS:Ce thin films for electroluminescent devices.Applied Surface Science, 1998, 133: 205-212. |
| [29] | ZOU Y Q, LI X J, XU J, et al.Different chemical valence of V element in the YVO4 crystal.Journal of Synthetic Crystals, 2003, 32(1): 27-30. |
| [30] | WANG Y F, WU Z L, RUAN Y F, et al. Spectroscopic properties of cerium doped YVO4 crystals and analysis on valence state of cerium ion. Acta Physica Sinica, 2012, 61(22): 228105(1)- 228105(8). |
| [31] | YANG Z Y, WEI Q, HAO Y.Investigations of lattice distortion and EPR parameters for YAG:V2+ laser crystal.Journal of Synthetic Crystals, 2005, 34(3): 491-495. |
| [32] | HUANG Y P.Theoretical studies of optical spectra and EPR parameters for V4+ in ThSiO4 crystal.Journal of Synthetic Crystals, 2008, 37(5): 1145-1147. |
| [33] | GREGORIO S D, GREENBLATT M, PIFER J H, et al.An ESR and optical study of V4+ in zircon-type crystals.The Journal of Chemical Physics, 1982, 76(6): 2931-2937. |
| [34] | TOMASZ G, ARTUR T.Up-conversion luminescence of GdOF: Yb3+, Ln3+ (Ln=Ho, Tm, Er) nanocrystals.Journal of Alloys and Compounds, 2016, 660: 235-243. |
| [1] | LI Yue, ZHANG Xuliang, JING Fangli, HU Zhanggui, WU Yicheng. Growth and Property of Ce3+-doped La2CaB10O19 Crystal [J]. Journal of Inorganic Materials, 2023, 38(5): 583-588. |
| [2] | WANG Zhaowu, JI Haipeng, WANG Feixiang, HOU Xinghui, YI Shasha, ZHOU Ying, CHEN Deliang. Valence State Control of Manganese in MgAl2O4:Mn4+ Phosphor by Varying the Al2O3 Crystal Form [J]. Journal of Inorganic Materials, 2021, 36(5): 513-520. |
| [3] | ZHOU Xiong, FANG Lizhi, HUANG Shuangwu, XIA Haiping, HU Jianxu, ZHANG Jianli, CHEN Baojiu. Ultraviolet and Near-infrared Luminescence of Ce 3+/Yb 3+ Co-doping LiLuF4 Single Crystal [J]. Journal of Inorganic Materials, 2020, 35(5): 556-560. |
| [4] | LI Sheng-Song, ZHENG Yong-Chao, MENG Shu-Lin, WU Li-Zhu, ZHONG Jin- Yi, ZHAO Chong-Lin. Core/Shell Quantum Dots and Au Nanoparticles Assembly for Effective Detection of Nerve Agent Mimic [J]. Journal of Inorganic Materials, 2019, 34(8): 893-898. |
| [5] | SHI Zhong-Xiang, WANG Jing, GUAN Xin. Multicolor Upconversion Emission Tuning of NaY(WO4)2: Dy3+ via Er3+ Doping [J]. Journal of Inorganic Materials, 2018, 33(5): 521-527. |
| [6] | ZHOU Ding, SHI Ying, FAN Ling-Cong, LIN De-Bao, SUN Ze-Qing, XU Jia-Yue. Fabrication and Optical Properties of Ce, Pr Co-doped LuAG Transparent Ceramics [J]. Journal of Inorganic Materials, 2016, 31(10): 1099-1102. |
| [7] |
PENG Xia, LI Shu-Xing, LIU Xue-Jian, HUANG Yi-Hua, HUANG Zheng-Ren, LI Hui-Li.
Syntheses and Photoluminescence Properties of Eu2+/Tb3+ Doped Sr2Si5N8 Phosphors [J]. Journal of Inorganic Materials, 2015, 30(4): 397-400. |
| [8] | XIANG Jun-Tao, DU Peng, LUO Lai-Hui, FANG Yi-Quan, ZHAO Xue-Yang, HU Xu-Bo, CHEN Hong-Bing. Growth and Characterization of Er3+-doped Relaxor-based Ferroelectric Crystal PMNT [J]. Journal of Inorganic Materials, 2015, 30(2): 135-140. |
| [9] | LUO Jia-Liang, WU Yun-Tao, REN Guo-Hao. Luminescence Property and Energy Transfer of Ce3+, Eu3+ Co-doped Lu3Al5O12 Polycrystals [J]. Journal of Inorganic Materials, 2014, 29(11): 1211-1217. |
| [10] | ZHANG Shou-Chao, RUAN Yong-Feng, JIA Guo-Zhi, FENG Zhi-Hui, LIU Zhi-Peng, PEI Li-Bin. Blue-emitting Properties of Ce3+ Doped YVO4 under Ultraviolet Excitation [J]. Journal of Inorganic Materials, 2014, 29(10): 1067-1072. |
| [11] | WANG Sen, ZHOU Ya-Xun, DAI Shi-Xun, WANG Xun-Si, SHEN Xiang, CHEN Fei-Fei. Influence of High Phonon Energy Oxide on Spectroscopic Properties of Er3+/Ce3+ Co-doped Tellurite Glasses [J]. Journal of Inorganic Materials, 2012, 27(8): 865-870. |
| [12] | WEI Shu-Lin, XU Yin-Sheng, ZHANG Pei-Qing, CHEN Fei-Fei, NIE Qiu-Hua, XU Tie-Feng, DAI Shi-Xun. 2.00 μm Emission Properties of Tm3+/Ho3+ Co-doped Chalcohalide Glasses [J]. Journal of Inorganic Materials, 2012, 27(7): 711-715. |
| [13] | LI Lei, ZHU Kai, ZHANG Li, CHEN Da-Zhou. Preparation and Photophysical Properties of Materials Obtained by Co-intercalation of 4-MAA and 2-NSA into Mg-Al-LDH [J]. Journal of Inorganic Materials, 2012, 27(2): 122-128. |
| [14] | HU Yue-Bo1,2,3, QIU Jian-Bei2, ZHOU Da-Li1, YANG Zhen-Wen2, SONG Zhi-Guo2. Characteristics and Mechanism of Up-conversion Luminescence in Er3+/Yb3+/Tb3+ Co-doped Oxyfluorogermanate Glasses [J]. Journal of Inorganic Materials, 2010, 25(5): 551-556. |
| [15] | CHEN Shi, ZHOU Guo-Hong, ZHANG Hai-Long, YANG Yan, WANG Shi-Wei. Synthesis and Optical Properties of Core Shell NaYF4: Yb3+, Er3+ / SiO2 Particles [J]. Journal of Inorganic Materials, 2010, 25(11): 1128-1132. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||