
Journal of Inorganic Materials ›› 2016, Vol. 31 ›› Issue (6): 667-672.DOI: 10.15541/jim20150607
• Orginal Article • Previous Articles
HAN Dan-Dan1, JING Xiao-Yan2, XU Peng-Cheng1, TAN Ao1, CHENG Zhen-Yu1
Received:2015-12-04
Published:2016-06-20
Online:2016-05-19
Supported by:CLC Number:
HAN Dan-Dan, JING Xiao-Yan, XU Peng-Cheng, TAN Ao, CHENG Zhen-Yu. Optimizing the Charge Transfer Process by Synthesizing NiO Microspheres on Ni Foam through Microwave-assisted Method[J]. Journal of Inorganic Materials, 2016, 31(6): 667-672.
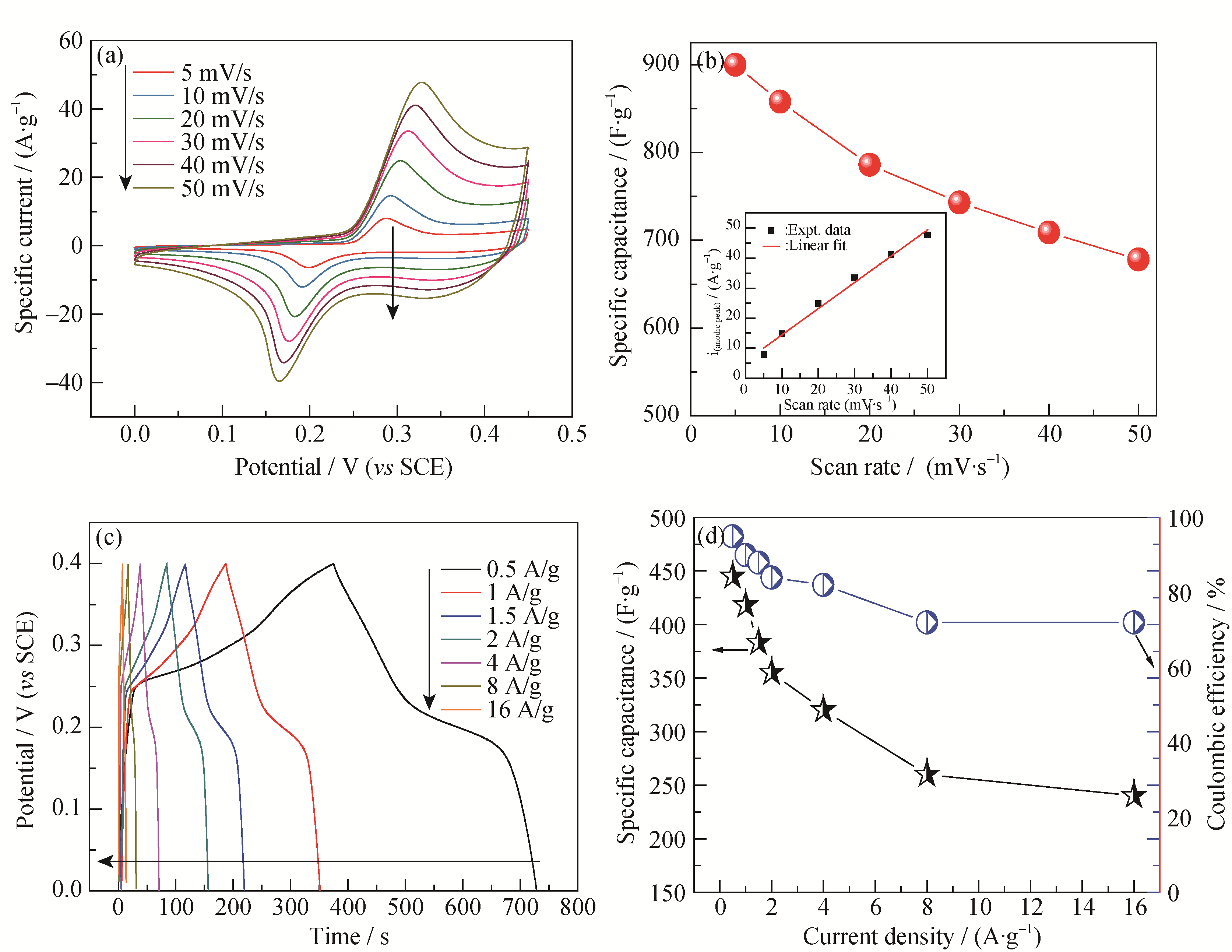
Fig. 4 Electrochemical properties of NW-NiO electrode. (a) CVs of hierachical NW-NiO electrode at the scan rates of 5, 10, 20, 30, 40, 50 mV/s, respectively; (b) Specific capacitance as a function of scan rate, the inset of (b) Shows the linearity of anodic current density with scan rate; (c) Charge and discharge curves of samples at different current density; (d) Specific capacitances and coulombic efficiency as function of discharge current densities of NW-NiO electrode
| Electrode structure | Electrolyte | Rct /Ω | Reference |
|---|---|---|---|
| Hierarchical NiO sphere/Ni | KOH (2mol·L-1) | 0.53 | This work |
| Porous NiO sheet/Ni | KOH (6 mol·L-1) | ≈10 | [23] |
| Co-doped NiO film/FTO | KOH (1 mol·L-1) | 12.03 | [24] |
| NiO/CuO nanoflower/ Ni | KOH (6 mol·L-1) | ≈15 | [25] |
| NiO/MWCNT thin film/stainless steel | KOH (2 mol·L-1) | 1.72 | [26] |
Table 1 Compared Rct for different structured NiO electrodes reported in previous literature
| Electrode structure | Electrolyte | Rct /Ω | Reference |
|---|---|---|---|
| Hierarchical NiO sphere/Ni | KOH (2mol·L-1) | 0.53 | This work |
| Porous NiO sheet/Ni | KOH (6 mol·L-1) | ≈10 | [23] |
| Co-doped NiO film/FTO | KOH (1 mol·L-1) | 12.03 | [24] |
| NiO/CuO nanoflower/ Ni | KOH (6 mol·L-1) | ≈15 | [25] |
| NiO/MWCNT thin film/stainless steel | KOH (2 mol·L-1) | 1.72 | [26] |
| [1] | SIMON P, GOGOTSI Y.Materials for electrochemical capacitors.Nat. Mater., 2008, 7(11): 845-854. |
| [2] | WANG G, ZHANG L, ZHANG J.A review of electrode materials for electrochemical supercapacitors.Chem. Soc. Rev., 2012, 41(2): 797-828. |
| [3] | ZHANG G Q, LOU X W.General solution growth of mesoporous NiCo2O4 nanosheets on various conductive substrates as high-performance electrodes for supercapacitors. Adv. Mater., 2013, 25(7): 975-979. |
| [4] | ZHU G X, XI C Y, SHEN M Q, et al.Nanosheet-based hierarchical Ni2(CO3)(OH)2 microspheres with meak crystallinity for high-performance supercapacitor.ACS Appl. Mater. Interfaces, 2014, 6(19): 17208-17214. |
| [5] | WANG B, HE X Y, LI H P, et al.Optimizing the charge transfer process by designing Co3O4@PPy@MnO2 ternary core-shell composite.J. Mater. Chem. A, 2014, 2(32): 12968-12973. |
| [6] | HU C C, CHEN W C, CHANG K H.How to achieve maximum utilization of hydrous ruthenium oxide for supercapacitors.J. Electrochem. Soc., 2004, 151(2): A281-A290. |
| [7] | WANG J G, KANG F Y, WEI B Q.Engineering of MnO2-based nanocomposites for high-performance supercapacitors.Prog. Mater. Sci., 2015, 74(1): 51-124. |
| [8] | CHODANKAR N R, DUBAL D P, GUND G S, et al.Flexible all-solid-state MnO2 thin films based symmetric supercapacitors.Electrochim. Acta, 2015, 165(20): 338-347. |
| [9] | YAN X Y, TONG X L, WANG J, et al.Synthesis of mesoporous NiO nanoflake array and its enhanced electrochemical performance for supercapacitor application.J. Alloys Compd., 2014, 593(25): 184-189. |
| [10] | HU C C, CHEN J C, CHANG K H.Cathodic deposition of Ni(OH)2 and Co(OH)2 for asymmetric supercapacitors: importance of the electrochemical reversibility of redox couples.J. Power Sources, 2013, 221(1): 128-133. |
| [11] | YANG W L, GAO Z, MA J, et al.Hierarchical NiCo2O4@NiO core-shell heterostructured nanowire arrays on carbon cloth for a high-performance flexible all-solid-state electrochemical capacitor.J. Mater. Chem. A, 2014, 2(5): 1448-1457. |
| [12] | LI Y H, ZHANG Y F, LI Y J, et al.Unveiling the dynamic capacitive storage mechanism of Co3O4 @NiCo2O4 hybrid nanoelectrodes for supercapacitor applications.Electrochim. Acta, 2014, 145(1): 177-184. |
| [13] | CAI D P, HUANG H, WANG D D, et al.High-performance supercapacitor electrode based on the unique ZnO@Co3O4 core/shell hetero structures on nickel foam.ACS Appl. Mater. Interfaces, 2014, 6(18): 15905-15912. |
| [14] | WU J B, LI Z G, HUANG X H, et al.Porous Co3O4/NiO core/shell nanowire array with enhanced catalytic activity for methanol electro- oxidation.J. Power Sources, 2013, 224(1): 1-5. |
| [15] | YANG M H, LEE K G, LEE S J, et al.Three dimensional expanded grapheme metal oxide film via solid state microwave irradiation for aqueous asymmetric supercapacitors.ACS Appl. Mater. Interfaces, 2015, 7(40): 22364-22371. |
| [16] | HU X, GONG J, ZHANG L, et al.Continuous size tuning of monodisperse ZnO colloidal nanocrystal clusters by a microwave-polyol process and their application for humidity sensing.Adv. Mater., 2008, 20(24): 4845-4850. |
| [17] | YAN H L, ZHANG D Y, XU J Y, et al.Solution growth of NiO nanosheets supported on Ni foam as high-performance electrodes for super capaci- tors.Nanoscale Res. Lett., 2014, 9: 424-430. |
| [18] | QING X X, LIU S Q, HUANG K L, et al.Facile synthesis of Co3O4 nanoflowers grown on Ni foam with superior electrochemical performance.Electrochim. Acta, 2011, 56(14): 4985-4991. |
| [19] | BAGHBANZADEH M, CARBONE L, COZZOLI P D, et al.Microwave-assisted synthesis of colloidal inorganic nanocrystals.Angew. Chem. Int. Ed., 2011, 50(48): 11312-11359. |
| [20] | HSU H Y, CHANG K H, SALUNKHE R R, et al.Synthesis and characterization of mesoporous Ni-Co oxy-hydroxides for pseudocapacitor application.Electrochim. Acta, 2013, 94(1): 104-112. |
| [21] | AMADE R, JOVER E, CAGLAR B, et al.Optimization of MnO2/vertically aligned carbon nanotube composite for supercapacitor application.J. Power Sources, 2011, 196(13): 5779-5783. |
| [22] | MOTASEMI F, AFZAL M T.A review on the microwave-assisted pyrolysis technique.Renewable Sustainable Energy Rev., 2013, 28: 317-330. |
| [23] | YANG L, QIAN L, TIAN X Q, et al.Hierarchically porous nickel oxide nanosheets grown on nickel foam prepared by one-step in situ anodization for high-performance supercapacitors.Chem. Asian J., 2014, 9(6): 1579-1585. |
| [24] | ZHANG J H, CAI G F, ZHOU D, et al.Co-doped NiO nanoflake array films with enhanced electro chromic properties.J. Mater. Chem. C, 2014, 2(34): 7013-7021. |
| [25] | HUANG M, LI F, ZHANG Y X, et al.Hierarchical NiO nanoflake coated CuO flower core-shell nanostructures for supercapacitor.Ceram. Int., 2014, 40(4): 5533-5538. |
| [26] | GUND G S, DUBAL D P, SHINDE S S, et al.Architectured morphologies of chemically prepared NiO/MWCNTs nanohybrid thin films for High Performance Supercapacitors.ACS Appl. Mater. Interfaces, 2014, 6(5): 3176-3188. |
| [1] | LIU Fangfang, CHUAN Xiuyun, YANG Yang, LI Aijun. Influence of N/S Co-doping on Electrochemical Property of Brucite Template Carbon Nanotubes [J]. Journal of Inorganic Materials, 2021, 36(7): 711-717. |
| [2] | ZHAN Jing,XU Changfan,LONG Yiyu,LI Qihou. Bi2Mn4O10: Preparation by Polyacrylamide Gel Method and Electrochemical Performance [J]. Journal of Inorganic Materials, 2020, 35(7): 827-833. |
| [3] | ZHU Zeyang,WEI Jishi,HUANG Jianhang,DONG Xiangyang,ZHANG Peng,XIONG Huanming. Preparation of ZnO Nanorods with Lattice Vacancies and Their Application in Ni-Zn Battery [J]. Journal of Inorganic Materials, 2020, 35(4): 423-430. |
| [4] | LI Xue-Lin, ZHU Jian-Feng, JIAO Yu-Hong, HUANG Jia-Xuan, ZHAO Qian-Nan. Manganese Dioxide Morphology on Electrochemical Performance of Ti3C2Tx@MnO2 Composites [J]. Journal of Inorganic Materials, 2020, 35(1): 119-125. |
| [5] | SUN Xiao-Lu,SONG Xiao-Fei,LIU Yan-Hua,WU Yue,CAI Yi-Bing,ZHAO Hong-Mei. Electrospun FeMnO3 Nanofibrous Mats: Preparation and Electrochemical Property [J]. Journal of Inorganic Materials, 2019, 34(7): 709-714. |
| [6] | WANG Jia-Hu, WANG Wen-Xin, DU Peng, HU Fang-Dong, JIANG Xiao-Lei, YANG Jian. Synthesis of Na3V2(PO4)2F3@V2O5-x as Cathode Material for Sodium-ion Battery [J]. Journal of Inorganic Materials, 2019, 34(10): 1097-1102. |
| [7] | FAN Guang-Xin, LIU Ze-Ping, WEN Yin, LIU Bao-Zhong. Surface Treatment on Structure and Property of LiNi0.8Co0.15Al0.05O2 by Silane Coupling Agent [J]. Journal of Inorganic Materials, 2018, 33(7): 749-755. |
| [8] | LUO Ling-Hong, HU Jia-Xing, CHENG Liang, XU Xu, WU Ye-Fan, LIN You-Chen. Performance of the Composite Cathode Ba0.5Sr0.5Co0.8Fe0.2O3-δ-Ce0.9Gd0.1O2-δ for Medium-low Temperature Solid Oxide Fuel Cell [J]. Journal of Inorganic Materials, 2018, 33(4): 441-446. |
| [9] | ZHANG Guo-Xiong, CHEN Yue-Mei, HE Zhen-Ni, LIN Chuan, CHEN Yi-Gang, GUO Hai-Bo. Surfactant Dependence of Nanostructured NiCo2S4 Films on Ni Foam for Superior Electrochemical Performance [J]. Journal of Inorganic Materials, 2018, 33(3): 289-294. |
| [10] | LIU Can-Jun, CHEN Shu, LI Jie. CdS/TiO2 Nanocrystalline Films: In-situ Synthesis and Photoelectrochemical Performance [J]. Journal of Inorganic Materials, 2018, 33(12): 1343-1348. |
| [11] | LIU Shuang-Yu, XU Li, CHEN Xin, HAN Yu, LIU Hai-Zhen, SHENG Peng, WANG Bo, ZHAO Guang-Yao. Graphene Loaded with Cluster Structural CoFe2O4 Particles and Its Li-Storage Performanc [J]. Journal of Inorganic Materials, 2017, 32(9): 904-908. |
| [12] | LI Si-Lin, TU Heng-Yong, YU Li-Jun. Performance of Nd2NiO4+δ-Ce0.8Gd0.2O2-δ Composite Cathodes for Intermediate Temperature Solid Oxide Fuel Cells [J]. Journal of Inorganic Materials, 2017, 32(5): 469-475. |
| [13] | LI Wei, ZHANG Yuan-Jie, WANG Xuan-Peng, NIU Chao-Jiang, AN Qin-You, MAI Li-Qiang. Synthesis and Electrochemical Performance of LiMn0.6Fe0.4PO4/C Cathode for Lithium-ion Batteries [J]. Journal of Inorganic Materials, 2017, 32(5): 476-482. |
| [14] | AO Xin, WU Wei-Xiang, WU Tian, WU Mei-Fen, WEN Zhao-Yin. Operating Temperature on Cathode Material and Electrochemical Performance of Na-NiCl2 Batteries [J]. Journal of Inorganic Materials, 2017, 32(12): 1243-1249. |
| [15] | WANG Hao, LI Lin, WANG Chun-Lei, WANG Qian, LIANG Chang-Hai, WANG Tong-Hua. Preparation and Electrochemical Performance of Polyimide-based Activated Carbons with High Surface Area [J]. Journal of Inorganic Materials, 2017, 32(11): 1181-1187. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||