
Journal of Inorganic Materials ›› 2016, Vol. 31 ›› Issue (4): 421-426.DOI: 10.15541/jim20150413
• Orginal Article • Previous Articles Next Articles
HU Xiao-Xia1, ZHAO Lin1, ZHAO Shu-Yu2, LI Rong3, XING Yan-Jun1
Received:2015-08-31
Revised:2015-12-13
Published:2016-04-20
Online:2016-03-25
About author:HU Xiao-Xia. E-mail:18817333895@163.com
Supported by:CLC Number:
HU Xiao-Xia, ZHAO Lin, ZHAO Shu-Yu, LI Rong, XING Yan-Jun. Microwave-assisted Preparation of Copper Hydroxyphosphate and Characterization of Photocatalysis under Visible Light[J]. Journal of Inorganic Materials, 2016, 31(4): 421-426.
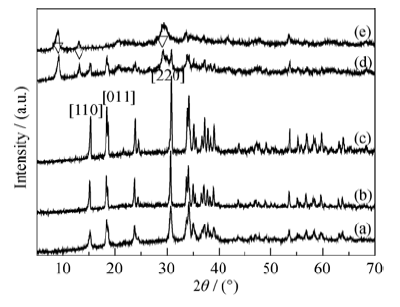
Fig. 4 XRD patterns of Cu2(OH)PO4 synthesized with different [PO43-] concentrations (n(Cu)/n(P) =2) (a) 0.0025 mol/L; (b) 0.005 mol/L; (c) 0.010 mol/L; (d) 0.025 mol/L; (e) 0.100 mol/L
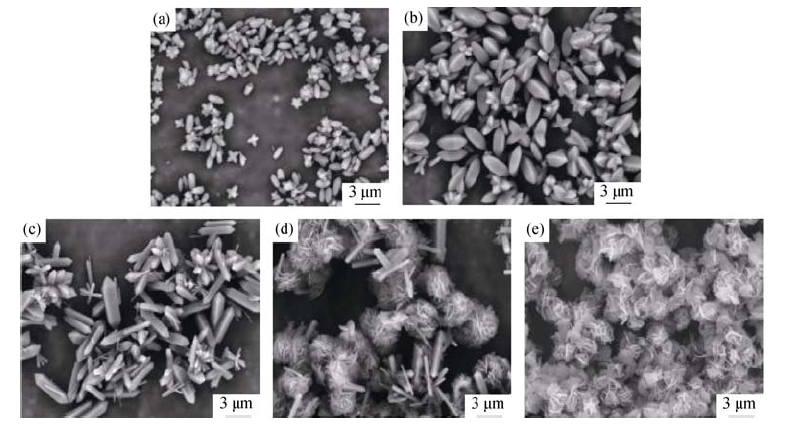
Fig. 5 SEM images of Cu2(OH)PO4 synthesized with different [PO43-] concentrations (n(Cu)/n(P) =2) (a) 0.0025 mol/L; (b) 0.005 mol/L; (c) 0.010 mol/L; (d) 0.025 mol/L; (e) 0.100 mol/L
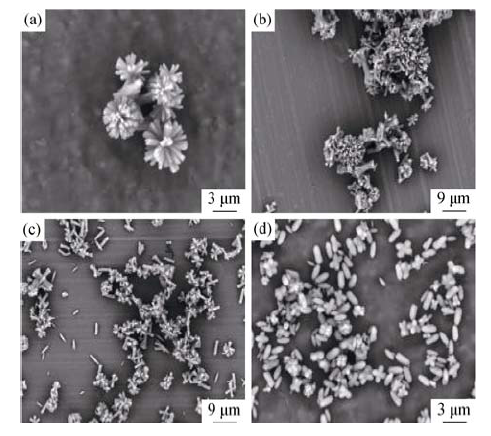
Fig. 6 SEM images of Cu2(OH)PO4 synthesized without and with different surfactants (a) Without surfactant; (b) CTAB (CMC, 0.04 g); (c) PVP (m(PVP)/ [Cu2+]= 20, 0.64 g); (d) SDS (0.10 g)
| Test | [PO43-]/ (mol·L-1)* | Temperature/℃ | Time /min | Urea usage/g | SDS usage/g | Degradation efficiency /% |
|---|---|---|---|---|---|---|
| 1 | 0.0025 | 80 | 30 | 6.0 | 0.10 | 52.91 |
| 2 | 0.0050 | 80 | 30 | 6.0 | 0.10 | 51.06 |
| 3 | 0.0100 | 80 | 30 | 6.0 | 0.10 | 47.30 |
| 4 | 0.0025 | 80 | 30 | 3.0 | 0.05 | 45.41 |
| 5 | 0.0025 | 80 | 30 | 6.0 | 0.05 | 50.79 |
| 6 | 0.0025 | 80 | 30 | 9.0 | 0.05 | 68.11 |
| 7 | 0.0025 | 70 | 30 | 6.0 | 0.10 | 44.19 |
| 8 | 0.0025 | 90 | 30 | 6.0 | 0.10 | 49.68 |
| 9 | 0.0025 | 95 | 30 | 6.0 | 0.10 | 60.08 |
| 10 | 0.0025 | 80 | 15 | 6.0 | 0.10 | 45.21 |
| 11 | 0.0025 | 80 | 60 | 6.0 | 0.10 | 51.61 |
Table 1 Influence of preparation conditions on the photocatalytic degradation of MB
| Test | [PO43-]/ (mol·L-1)* | Temperature/℃ | Time /min | Urea usage/g | SDS usage/g | Degradation efficiency /% |
|---|---|---|---|---|---|---|
| 1 | 0.0025 | 80 | 30 | 6.0 | 0.10 | 52.91 |
| 2 | 0.0050 | 80 | 30 | 6.0 | 0.10 | 51.06 |
| 3 | 0.0100 | 80 | 30 | 6.0 | 0.10 | 47.30 |
| 4 | 0.0025 | 80 | 30 | 3.0 | 0.05 | 45.41 |
| 5 | 0.0025 | 80 | 30 | 6.0 | 0.05 | 50.79 |
| 6 | 0.0025 | 80 | 30 | 9.0 | 0.05 | 68.11 |
| 7 | 0.0025 | 70 | 30 | 6.0 | 0.10 | 44.19 |
| 8 | 0.0025 | 90 | 30 | 6.0 | 0.10 | 49.68 |
| 9 | 0.0025 | 95 | 30 | 6.0 | 0.10 | 60.08 |
| 10 | 0.0025 | 80 | 15 | 6.0 | 0.10 | 45.21 |
| 11 | 0.0025 | 80 | 60 | 6.0 | 0.10 | 51.61 |
| Runs | Degradation efficiency/% |
|---|---|
| 1 | 52.91 |
| 2 | 50.64 |
| 3 | 46.82 |
| 4 | 47.45 |
| 5 | 48.66 |
Table 2 Degradation of MB in consecutive runs using the recycled Cu2(OH)PO4
| Runs | Degradation efficiency/% |
|---|---|
| 1 | 52.91 |
| 2 | 50.64 |
| 3 | 46.82 |
| 4 | 47.45 |
| 5 | 48.66 |
| [1] | XIAO F S, SUN J M, MENG X J, et al.A novel catalyst of copper hydroxyphosphate with high activity in wet oxidation of aromatics.Applied Catalysis A: General, 2001, 207(1/2): 267-271. |
| [2] | MENG X J, LIN K F, YANG X Y, et al.Catalytic oxidation of olefins and alcohols by molecular oxygen under air pressure over Cu2(OH)PO4 and Cu4O(PO4)2 catalysts.Journal of Catalysis, 2003, 218(2): 460-464. |
| [3] | XIAO F S, SUN J M, MENG X J, et al.Synthesis and structure of copper hydroxyphosphate and its high catalytic activity in hydroxylation of phenol by H2O2.Journal of Catalysis, 2001, 199(2): 273-281. |
| [4] | MENG X J, LIN K F, SUN J M, et al.Catalytic epoxidation of styrene over copper hydroxyphosphate Cu2(OH)PO4.Catalysis Letters, 2001, 71(3): 241-244. |
| [5] | SREENIVASULU P, VISWANADHAM A, NANDAN D, et al.Synthesis and catalytic applications of amine interacted Cu2(OH) PO4 nanoplates (copper NPs) and tubes (copper NTs).RSC Advances, 2013, 3(3): 729-732. |
| [6] | ZHAN Y Z, LI H L, CHEN Y L.Copper hydroxyphosphate as catalyst for the wet hydrogen peroxide oxidation of azo dyes,Journal of Hazardous Materials, 2010, 180(1/2/3): 481-485. |
| [7] | CHO I S, KIM D W, LEE S, et al.Synthesis of Cu2PO4OH hierarchical superstructures with photocatalytic activity in visible light,Advanced Functional Materials, 2008, 18(15): 2154-2162. |
| [8] | MENG X J, XIAO F S.Novel copper phosphates with high catalytic activities under mild conditions. Acta Physico-Chimica Sinica, 2004, 20(S): 939-945. |
| [9] | CUI L, HUI K N, HUI K S, et al.Facile microwave-assisted hydrothermal synthesis of TiO2 nanotubes.Material Letter, 2012, 75(5): 175-178. |
| [10] | 付伟伟. Ti-V-Cu过渡金属化合物能源材料-催化材料的合成与制备. 长春, 吉林大学博士学位论文, 2013. |
| [11] | 沈启慧. 无机功能材料的合成方法研究. 长春, 吉林大学博士学位论文, 2008. |
| [12] | ZHANG X Y, DING Y B, TANG X Y, et al.Degradation of bisphenol A by hydrogen peroxide activated with CuFeO2 microparticles as a heterogeneous Fenton-like catalyst: efficiency, stability and mechanism.Chemical Engineering Journal, 2014, 236: 251-262. |
| [1] | WU Lin, HU Minglei, WANG Liping, HUANG Shaomeng, ZHOU Xiangyuan. Preparation of TiHAP@g-C3N4 Heterojunction and Photocatalytic Degradation of Methyl Orange [J]. Journal of Inorganic Materials, 2023, 38(5): 503-510. |
| [2] | MA Xinquan, LI Xibao, CHEN Zhi, FENG Zhijun, HUANG Juntong. BiOBr/ZnMoO4 Step-scheme Heterojunction: Construction and Photocatalytic Degradation Properties [J]. Journal of Inorganic Materials, 2023, 38(1): 62-70. |
| [3] | CHEN Hanxiang, ZHOU Min, MO Zhao, YI Jianjian, LI Huaming, XU Hui. 0D/2D CoN/g-C3N4 Composites: Structure and Photocatalytic Performance for Hydrogen Production [J]. Journal of Inorganic Materials, 2022, 37(9): 1001-1008. |
| [4] | XUE Hongyun, WANG Congyu, MAHMOOD Asad, YU Jiajun, WANG Yan, XIE Xiaofeng, SUN Jing. Two-dimensional g-C3N4 Compositing with Ag-TiO2 as Deactivation Resistant Photocatalyst for Degradation of Gaseous Acetaldehyde [J]. Journal of Inorganic Materials, 2022, 37(8): 865-872. |
| [5] | CHI Congcong, QU Panpan, REN Chaonan, XU Xin, BAI Feifei, ZHANG Danjie. Preparation of SiO2@Ag@SiO2@TiO2 Core-shell Structure and Its Photocatalytic Degradation Property [J]. Journal of Inorganic Materials, 2022, 37(7): 750-756. |
| [6] | WANG Xiaojun, XU Wen, LIU Runlu, PAN Hui, ZHU Shenmin. Preparation and Properties of Ag@C3N4 Photocatalyst Supported by Hydrogel [J]. Journal of Inorganic Materials, 2022, 37(7): 731-740. |
| [7] | LIU Xuechen, ZENG Di, ZHOU Yuanyi, WANG Haipeng, ZHANG Ling, WANG Wenzhong. Selective Oxidation of Biomass over Modified Carbon Nitride Photocatalysts [J]. Journal of Inorganic Materials, 2022, 37(1): 38-44. |
| [8] | ZHANG Xian, ZHANG Ce, JIANG Wenjun, FENG Deqiang, YAO Wei. Synthesis, Electronic Structure and Visible Light Photocatalytic Performance of Quaternary BiMnVO5 [J]. Journal of Inorganic Materials, 2022, 37(1): 58-64. |
| [9] | LIU Peng, WU Shimiao, WU Yunfeng, ZHANG Ning. Synthesis of Zn0.4(CuGa)0.3Ga2S4/CdS Photocatalyst for CO2 Reduction [J]. Journal of Inorganic Materials, 2022, 37(1): 15-21. |
| [10] | WANG Luping, LU Zhanhui, WEI Xin, FANG Ming, WANG Xiangke. Application of Improved Grey Model in Photocatalytic Data Prediction [J]. Journal of Inorganic Materials, 2021, 36(8): 871-876. |
| [11] | AN Weijia, LI Jing, WANG Shuyao, HU Jinshan, LIN Zaiyuan, CUI Wenquan, LIU Li, XIE Jun, LIANG Yinghua. Fe(III)/rGO/Bi2MoO6 Composite Photocatalyst Preparation and Phenol Degradation by Photocatalytic Fenton Synergy [J]. Journal of Inorganic Materials, 2021, 36(6): 615-622. |
| [12] | XIAO Xiang, GUO Shaoke, DING Cheng, ZHANG Zhijie, HUANG Hairui, XU Jiayue. CsPbBr3@TiO2 Core-shell Structure Nanocomposite as Water Stable and Efficient Visible-light-driven Photocatalyst [J]. Journal of Inorganic Materials, 2021, 36(5): 507-512. |
| [13] | XIONG Jinyan, LUO Qiang, ZHAO Kai, ZHANG Mengmeng, HAN Chao, CHENG Gang. Facilely Anchoring Cu nanoparticles on WO3 Nanocubes for Enhanced Photocatalysis through Efficient Interface Charge Transfer [J]. Journal of Inorganic Materials, 2021, 36(3): 325-331. |
| [14] | SHU Mengyang, LU Jialin, ZHANG Zhijie, SHEN Tao, XU Jiayue. CsPbBr3 Perovskite Quantum Dots/Ultrathin C3N4 Nanosheet 0D/2D Composite: Enhanced Stability and Photocatalytic Activity [J]. Journal of Inorganic Materials, 2021, 36(11): 1217-1222. |
| [15] | LIU Yaxin, WANG Min, SHEN Meng, WANG Qiang, ZHANG Lingxia. Bi-doped Ceria with Increased Oxygen Vacancy for Enhanced CO2 Photoreduction Performance [J]. Journal of Inorganic Materials, 2021, 36(1): 88-94. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||