
Journal of Inorganic Materials ›› 2016, Vol. 31 ›› Issue (2): 159-164.DOI: 10.15541/jim20150293
• Orginal Article • Previous Articles Next Articles
GAN Qiong-Zhi, WEN Xiao-Ling, DING Yi-Ming, OUYANG Jian-Ming
Received:2015-06-24
Revised:2015-08-19
Published:2016-02-20
Online:2016-01-15
About author:GAN Qiong-Zhi. E-mail: ganqiongzhi@163.com
Supported by:CLC Number:
GAN Qiong-Zhi, WEN Xiao-Ling, DING Yi-Ming, OUYANG Jian-Ming. Adsorption of Cetyltrimethylammonium Bromide on Different-sized Calcium Oxalate Monohydrate and Dihydrate Crystals[J]. Journal of Inorganic Materials, 2016, 31(2): 159-164.

Fig. 1 XRD patterns of COM (A) and COD crystals (B) with different sizes after CTAB adsorption for 24 h (a1, b1): 50 nm; (a2, b2): 100 nm; (a3, b3): 1 μm; (a4, b4): 3 μm; (a5, b5): 10 μm
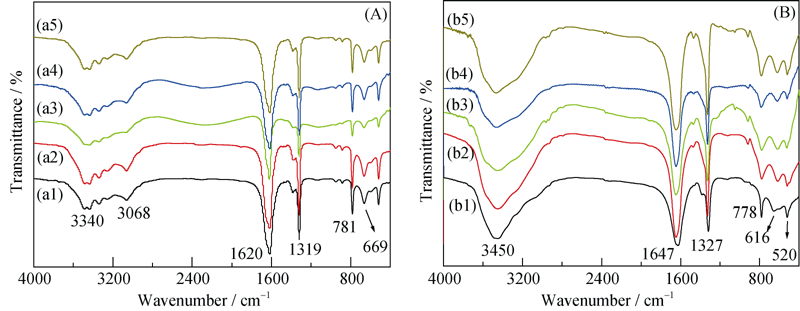
Fig. 2 FT-IR spectra of COM (A) and COD crystals (B) with different sizes after CTAB adsorption (a1, b1) 50 nm; (a2, b2) 100 nm; (a3, b3) 1 μm; (a4, b4) 3 μm; (a5, b5) 10 μm
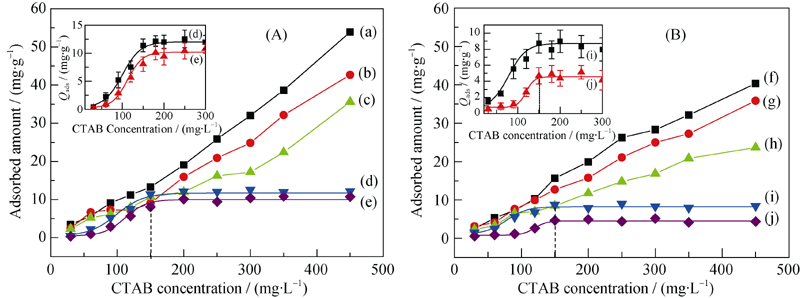
Fig. 3 Absorption amount changes of different sizes of COM (A) and COD (B) crystals in different concentrations of CTAB solution (a, f) 50 nm; (b, g) 100 nm; (c, h) 1 μm; (d, i) 3 μm; (e, j) 10 μm
| Crystal type | Crystal size (SEM characterization) | Specific surface area (SBET) / (m2·g-1) | Pore volume /(mm3·g-1) | Pore diameter /nm | Maximum adsorption amount (Qmax) /(mg·g-1) | Adsorption density (Γ#) / (N·nm-2) |
|---|---|---|---|---|---|---|
| COM-50 nm | 47.70± 6.20 nm | 26.30 | 49.20 | 7.49 | 53.90 | 3.39 |
| COM-100 nm | 92.10±10.40 nm | 14.70 | 37.30 | 10.10 | 42.70 | 4.78 |
| COM-1 μm | 0.91±0.22 μm | 13.60 | 37.70 | 11.10 | 35.60 | 4.31 |
| COM-3 μm | 2.65±0.43 μm | 1.51 | 3.30 | 6.71 | 12.60 | 13.80 |
| COM-10 μm | 9.67±1.76 μm | 0.83 | 1.30 | 6.37 | 11.10 | 22.10 |
| COD-50 nm | 44.10±8.70 nm | 40.80 | 95.70 | 9.37 | 40.30 | 1.63 |
| COD-100 nm | 98.30±8.10 nm | 21.40 | 40.90 | 7.63 | 35.80 | 2.76 |
| COD-1 μm | 0.92±0.31 μm | 9.12 | 31.50 | 13.80 | 23.70 | 4.29 |
| COD-3 μm | 3.41±0.57 μm | 1.36 | 1.20 | 4.61 | 8.97 | 10.90 |
| COD-10 μm | 9.58±0.97 μm | 0.80 | 1.10 | 5.71 | 5.13 | 10.60 |
Table 1 The maximum adsorption amount and adsorption density of CTAB on different sizes of COM and COD crystals at different surface areas, pore volumes and pore diameters
| Crystal type | Crystal size (SEM characterization) | Specific surface area (SBET) / (m2·g-1) | Pore volume /(mm3·g-1) | Pore diameter /nm | Maximum adsorption amount (Qmax) /(mg·g-1) | Adsorption density (Γ#) / (N·nm-2) |
|---|---|---|---|---|---|---|
| COM-50 nm | 47.70± 6.20 nm | 26.30 | 49.20 | 7.49 | 53.90 | 3.39 |
| COM-100 nm | 92.10±10.40 nm | 14.70 | 37.30 | 10.10 | 42.70 | 4.78 |
| COM-1 μm | 0.91±0.22 μm | 13.60 | 37.70 | 11.10 | 35.60 | 4.31 |
| COM-3 μm | 2.65±0.43 μm | 1.51 | 3.30 | 6.71 | 12.60 | 13.80 |
| COM-10 μm | 9.67±1.76 μm | 0.83 | 1.30 | 6.37 | 11.10 | 22.10 |
| COD-50 nm | 44.10±8.70 nm | 40.80 | 95.70 | 9.37 | 40.30 | 1.63 |
| COD-100 nm | 98.30±8.10 nm | 21.40 | 40.90 | 7.63 | 35.80 | 2.76 |
| COD-1 μm | 0.92±0.31 μm | 9.12 | 31.50 | 13.80 | 23.70 | 4.29 |
| COD-3 μm | 3.41±0.57 μm | 1.36 | 1.20 | 4.61 | 8.97 | 10.90 |
| COD-10 μm | 9.58±0.97 μm | 0.80 | 1.10 | 5.71 | 5.13 | 10.60 |
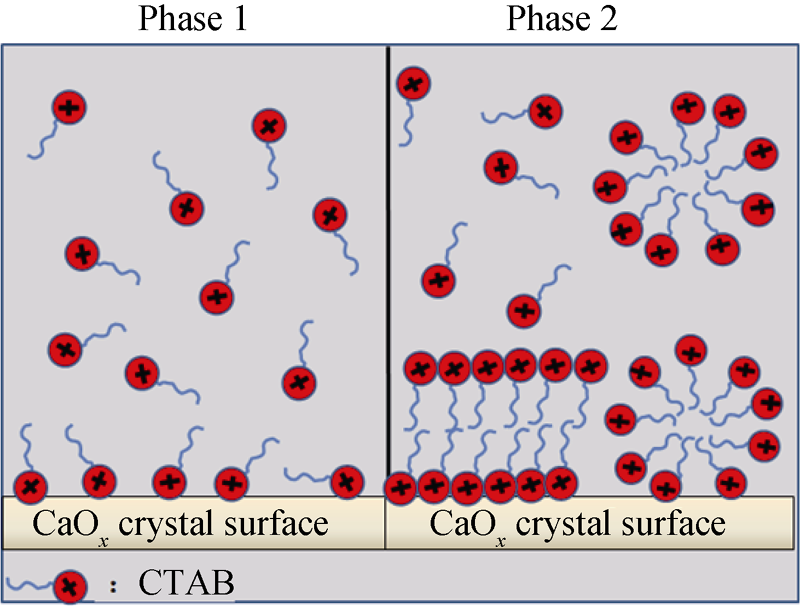
Fig. 4 Diagrams of CTAB molecules adsorbed on the surface of calcium oxalate crystals Phase 1: CTAB molecules formed monolayer absorption on the surface of calcium oxalate crystals Phase 2: CTAB molecules completely covered on crystal surface with bilayer or micelle
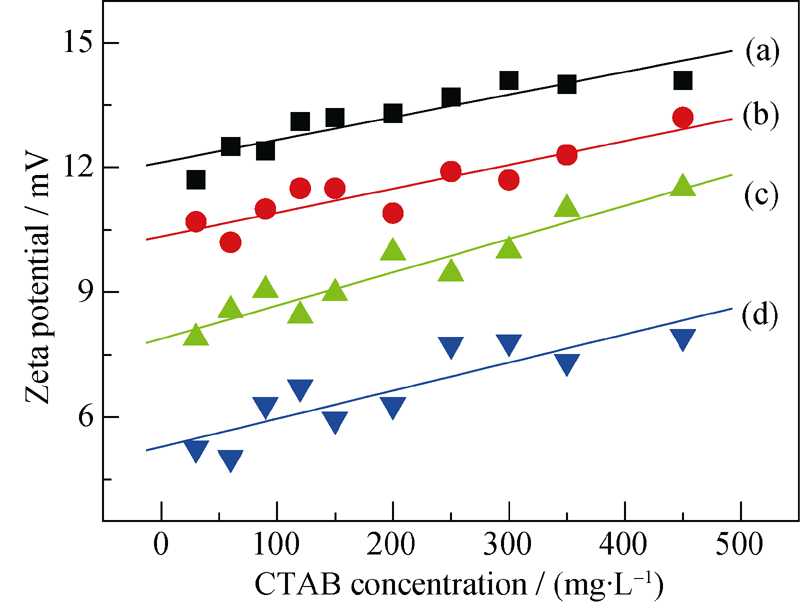
Fig. 5 Zeta potential change of different sizes of COM and COD crystals in different c (CTAB) (a) COM-50 nm; (b) COD-50 nm; (c) COM-3 μm; (d) COD-3 μm
| [1] | FARMANESH S, RAMAMOORTHY S, CHUNG J, et al.Specificity of growth inhibitors and their cooperative effects in calcium oxalate monohydrate crystallization.J. Am. Chem. Soc., 2014, 136(1): 367-376. |
| [2] | THURGOOD L A, GROVER P K, RYALL R L.High calcium concentration and calcium oxalate crystals cause significant inaccuracies in the measurement of urinary ostepontin by enzyme linked immunosorbent assay.Urol. Res., 2008, 36: 103-110. |
| [3] | GROHE B, O’YOUNG J, IONESCU D A, et al. Control of calcium oxalate crystal growth by face-specific adsorption of an osteopontin phosphopeptide.J. Am. Chem. Soc., 2007, 129: 14946-14951. |
| [4] | FISCHER V, LANDFESTER K, MUNOZ-ESPÍ R.Stabilization of calcium oxalate metastable phases by oligo(L-glutamic acid): effect of peptide chain length.Cryst. Growth Des., 2011, 11: 1880-1890. |
| [5] | GRASES F, MARCH J G.The crystallization of calcium oxalate in the presence of amino acids.J. Cryst. Growth, 1988, 87: 299-304. |
| [6] | SHENG X X, JUNG T, WESSON J A, et al.Adhesion at calcium oxalate crystal surfaces and the effect of urinary constituents.Proc. Nat. Acad. Sci., USA, 2005, 102(2): 267-272. |
| [7] | POON N W, GOHEL M D I. Urinary glycosaminoglycans and glycoproteins in a calcium oxalate crystallization system. Carbihyd. Res., 2012, 347: 64-68. |
| [8] | WALTON R C, KAVANAGH J P, HEYWOOD B R, et al.The association of different urinary proteins with calcium oxalate hydromorphs. Evidence for non-specific interactions.Biochim. Biophys. Acta, 2005, 1723(1/2/3): 175-183. |
| [9] | SUN X Y, OUYANG J M, ZHU W Y, et al.Size-dependent toxicity and interactions of calcium oxalate dihydrate crystal on Vero renal epithelial cells.J. Mater. Chem. B, 2015, 3: 1864-1878. |
| [10] | SASO L, GRIPPA E, GATTO M T, et al.Inhibition of calcium oxalate precipitation by bile salts. Int. J. Urol., 2001, 8(3): 124-127. |
| [11] | TUNIK L, FUREDI-MILHOFER H, GARTI N.Adsorption of sodium diisooctyl sulfosuccinate onto calcium oxalate crystals.Langmuir, 1998, 14: 3351-3355. |
| [12] | SUN X Y, OUYANG J M, LIU A J, et al.Preparation, characterization, and in vitro cytotoxicity of COM and COD crystals with various sizes.Mater. Sci. Eng. C, 2015, 57: 147-156. |
| [13] | CHEBOTAREV A N, PALADENKO T V, SHCHERBAKOVA T M.Adsorption-photometric determination of cationic surfactant traces.J. Anal. Chem., 2004, 59(4): 309-313. |
| [14] | KING M, MCCLURE W F, ANDREWS L C.Powder diffraction file alphabetic index, inorganic phases-organic phases. International Center for Diffraction Data: Newtown Square, PA, 1992. |
| [15] | SELVARAJU R, THIRUPPATHI G, RAJA A.FT-IR spectral studies on certain human urinary stones in the patients of rural area.Spectrochim. Acta A, 2012, 93: 260-265. |
| [16] | OUYANG J M, DUAN L, TIEKE B.Effects of carboxyl acids on the crystal growth of calcium oxalate nanoparticles in lecithin- water liposome systems. Langmuir, 2003, 19(21): 8980-8985. |
| [17] | STOCKER I N, MILLER K L, WELBOURN R J, et al.Adsorption of aerosol-OT at the calcite/water interface-comparison of the sodium and calcium salts. J. Colloid Interf. Sci., 2014, 418: 140-146. |
| [18] | WALSH R B, WU B, HOWARD S C, et al.Surface forces between titanium dioxide surfaces in the presence of cationic surfactant as a function of surfactant concentration, electrolyte concentration, and pH.Langmuir, 2014, 30(10): 2789-2798. |
| [19] | WEN Q, LI C, CAI Z, et al.Study on activated carbon derived from sewage sludge for adsorption of gaseous formaldehyde.Bioresour. Technol., 2011, 102(2): 942-947. |
| [20] | WANG X, JIANG Z Y, XIE Z X, et al.High-energy-surface engineered metal oxide micro- and nanocrystallites and their applications.Acounts Chem. Res., 2014, 42(7): 308-318. |
| [21] | RNSARI R, SHAHABODINI A, FAGHIH SHOJAEI M, et al.On the bending and buckling behaviors of mindlin nanoplates considering surface energies.Physica E, 2014, 57: 126-137. |
| [22] | CHUNG T H, WU S H, YAO M, et al.The effect of surface charge on the uptake and biological function of mesoporous silica nanoparticles in 3T3-L1 cells and human mesenchymal stem cells. Biomaterials, 2007, 28: 2959-2966. |
| [23] | SIKIRIC M, FILIPOVIC-VINCEKOVIC N, BABIC-IVANCIC V, et al.Interactions in calcium oxalate hydrate/surfactant systems.J. Colloid Interf. Sci., 1999, 212: 384-389. |
| [24] | WEI X X, YANG J, LI Z Y, et al.Comparison investigation of the effects of ionic surfactants on the crystallization behavior of calcium oxalate: From cationic to anionic surfactant.Colloids Surf. A, 2012, 401: 107-115. |
| [1] | LIANG Pei, XING Song, SHU Hai-Bo, ZHANG Lin, HU Chen-Li. Analogous Three-dimensional MoS2/Graphene Composites for Reversible Li Storage [J]. Journal of Inorganic Materials, 2016, 31(6): 575-580. |
| [2] | GAN Qiong-Zhi, ZHANG Chong-Yu, OUYANG Jian-Ming. Effect of Crystal Size of Calcium Oxalate Dihydrate on Cytotoxicity of African Green Monkey Kidney Epithelial Cells [J]. Journal of Inorganic Materials, 2016, 31(3): 317-323. |
| [3] | YAO Xiu-Qiong, HE Jie-Yu, YANG Jin, HOU Shan-Hua, OUYANG Jian-Ming. Aggregation of Urinary Microcrystallines with Different Sizes [J]. Journal of Inorganic Materials, 2012, 27(4): 343-347. |
| [4] | YANG Jin, LI Jun-Jun, YUAN Huan-Xin, OUYANG Jian-Ming. Regulation of Potassium Aminocarboxylates on Calcium Oxalate Crystal Growth and Its Relationship with Molecular Structure [J]. Journal of Inorganic Materials, 2010, 25(11): 1185-1190. |
| [5] | DENG Sui-Ping,DENG Lan-Qing,YU Hai-Yan,OUYANG Jian-Ming. Modulation of Polyacrylamide on Phases Compositions of Calcium Oxalate in Aqueous Solutions [J]. Journal of Inorganic Materials, 2007, 22(3): 427-431. |
| [6] | LIU Yi-Ming,YUAN Huan-Xin,OUYANG Jian-Ming. Modulation of Mixed Potassium Carboxylates on Crystallization of Calcium Oxalate Crystals in Gel System [J]. Journal of Inorganic Materials, 2007, 22(1): 75-78. |
| [7] | ZHANG Bing-Jian,YIN Hai-Yan,CHEN De-Yu,SHEN Zhong-Yue,LU Huan-Ming. Bioinorganic Material─The Crude Ca-oxalate Conservation Film on Historic Stone [J]. Journal of Inorganic Materials, 2001, 16(4): 752-756. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||