
Journal of Inorganic Materials ›› 2016, Vol. 31 ›› Issue (1): 14-20.DOI: 10.15541/jim20150295
• Orginal Article • Previous Articles Next Articles
LU Shu-Pei1, FENG Li-Li2, QI Lin1, WANG Li-Li2, QI Xing-Yi1
Received:2015-06-29
Revised:2015-09-09
Published:2016-01-20
Online:2015-12-15
About author:LU Shu-Pei. E-mail: sigmalsp@buaa.edu.cn
CLC Number:
LU Shu-Pei, FENG Li-Li, QI Lin, WANG Li-Li, QI Xing-Yi. Chemical Kinetics of Disproportionation Decomposition of tert-Butyl Hydroperoxide Catalyzed by Buserite-type Manganese Oxides[J]. Journal of Inorganic Materials, 2016, 31(1): 14-20.
| Me-buserite | Molecular formula | SBET / (m2·g-1) |
|---|---|---|
| Mg-buserite | Na0.03Mg3.99Mn14.00O30.11·19.05H2O | 61 |
| Co-buserite | Na0.02Mg3.29Co0.54Mn14.00O29.54·19.56H2O | 63 |
| Ni-buserite | Na0.03Mg1.32Ni1.43Mn14.00O28.68·14.17H2O | 57 |
| Cu-buserite | Na0.03Mg1.32Cu3.40Mn14.00O31.23·16.43H2O | 56 |
Table 1 Molecular formulas and BET surface areas of Me-buserites catalysts
| Me-buserite | Molecular formula | SBET / (m2·g-1) |
|---|---|---|
| Mg-buserite | Na0.03Mg3.99Mn14.00O30.11·19.05H2O | 61 |
| Co-buserite | Na0.02Mg3.29Co0.54Mn14.00O29.54·19.56H2O | 63 |
| Ni-buserite | Na0.03Mg1.32Ni1.43Mn14.00O28.68·14.17H2O | 57 |
| Cu-buserite | Na0.03Mg1.32Cu3.40Mn14.00O31.23·16.43H2O | 56 |
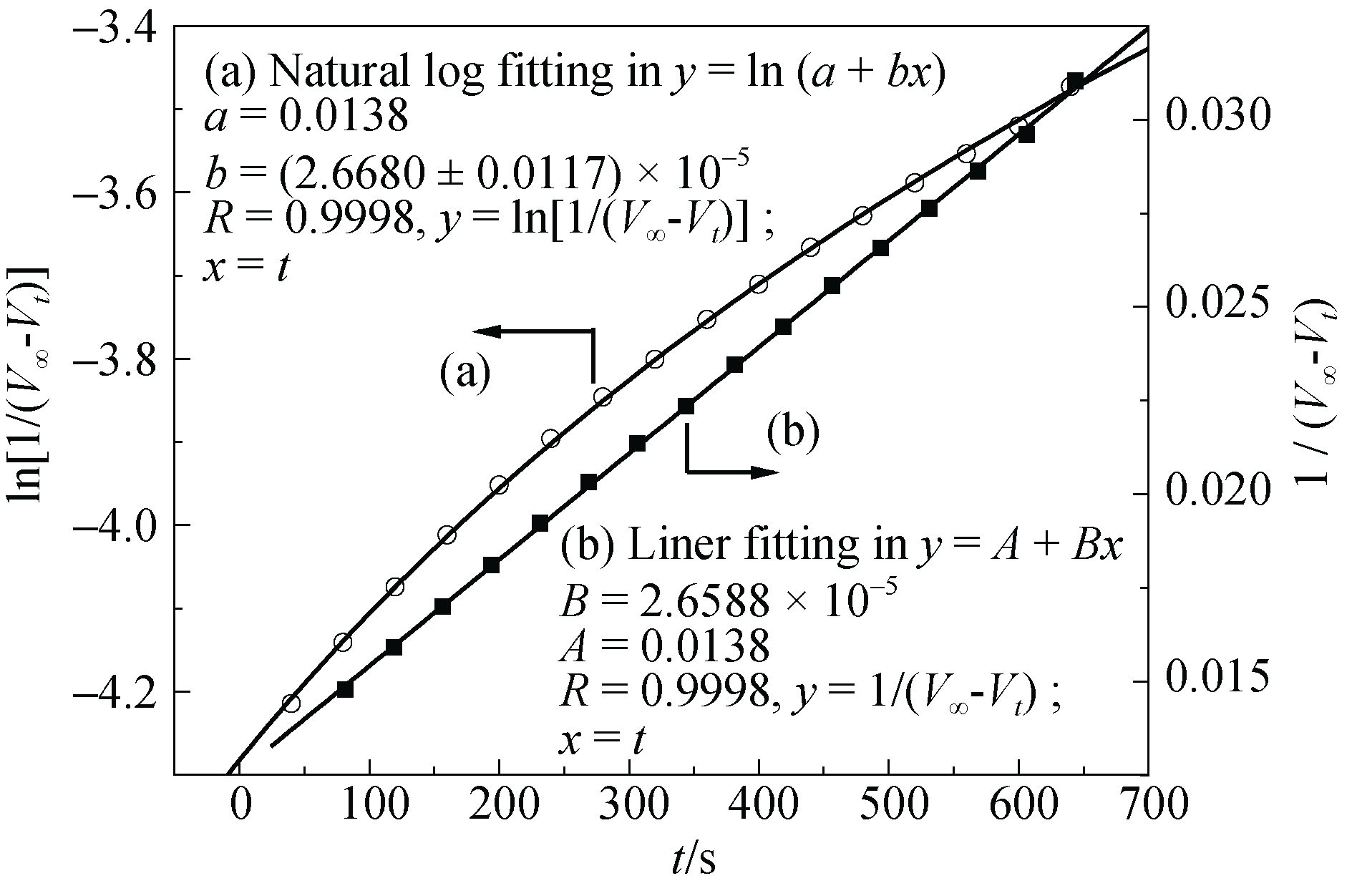
Fig. 2 Two kinetic fittings of the Vt and V∞ data (catalyst: Cu-buserite) (a) the natural log fitting of ln[1/(V∞ - Vt)] versus t; (b) the linear fitting of 1/(V∞ - Vt) versus t. The line of (b) here is that of (b) in Fig. 3 and for the reaction conditions, also see those shown in Fig.3
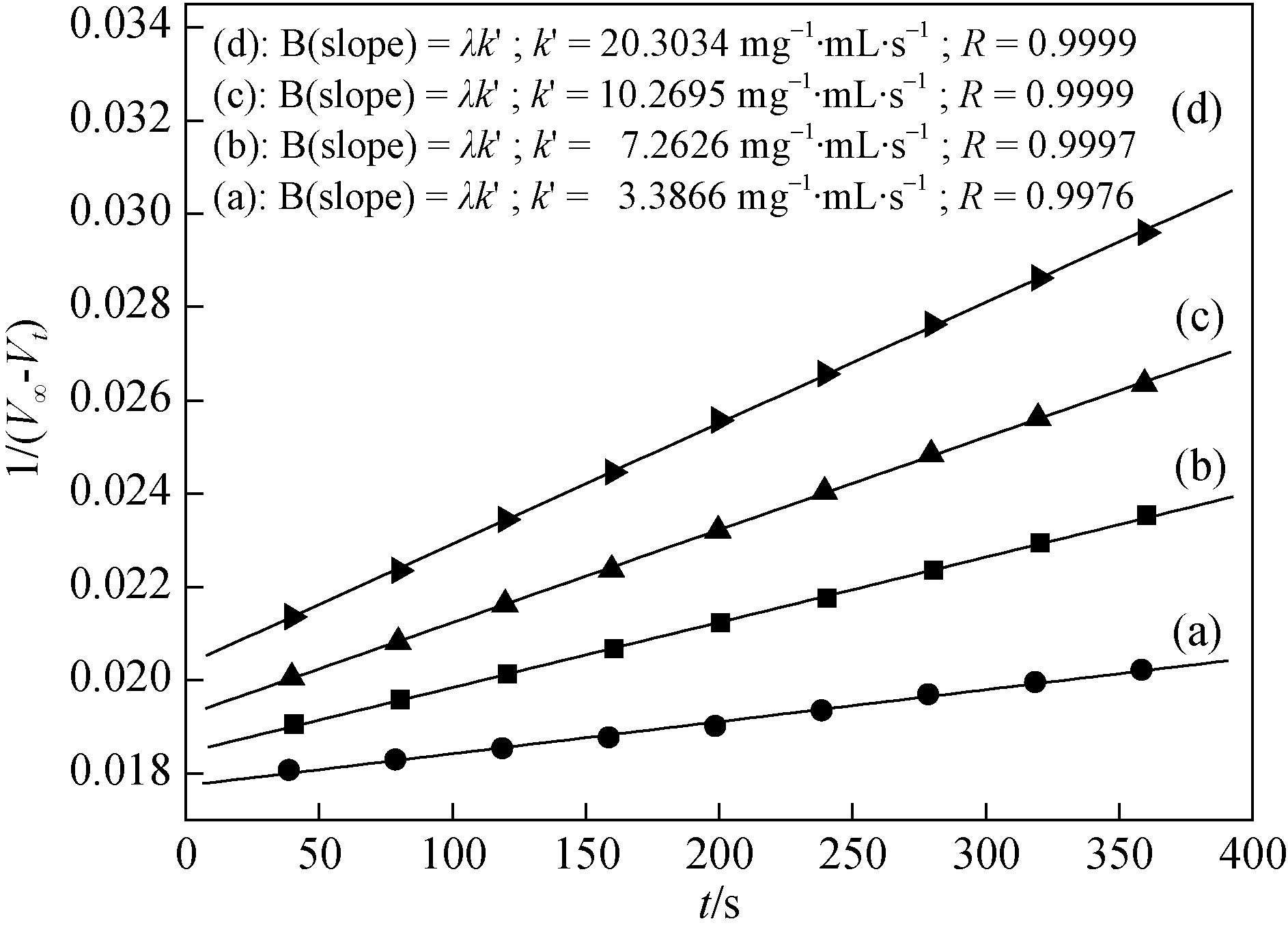
Fig. 3 Plots of 1/(V∞ - Vt) versus t obtained with different [Cu-buserite]s Reaction conditions: T 338 K, TBHP (65wt% in H2O) 1.00 mL, [Cu-buserite]a 1.67 mg/mL, [Cu-buserite]b 3.33 mg/mL, [Cu- buserite]c 5.00 mg/mL, [Cu-buserite]d 8.33 mg/mL, acetonitrile 5.00 mL
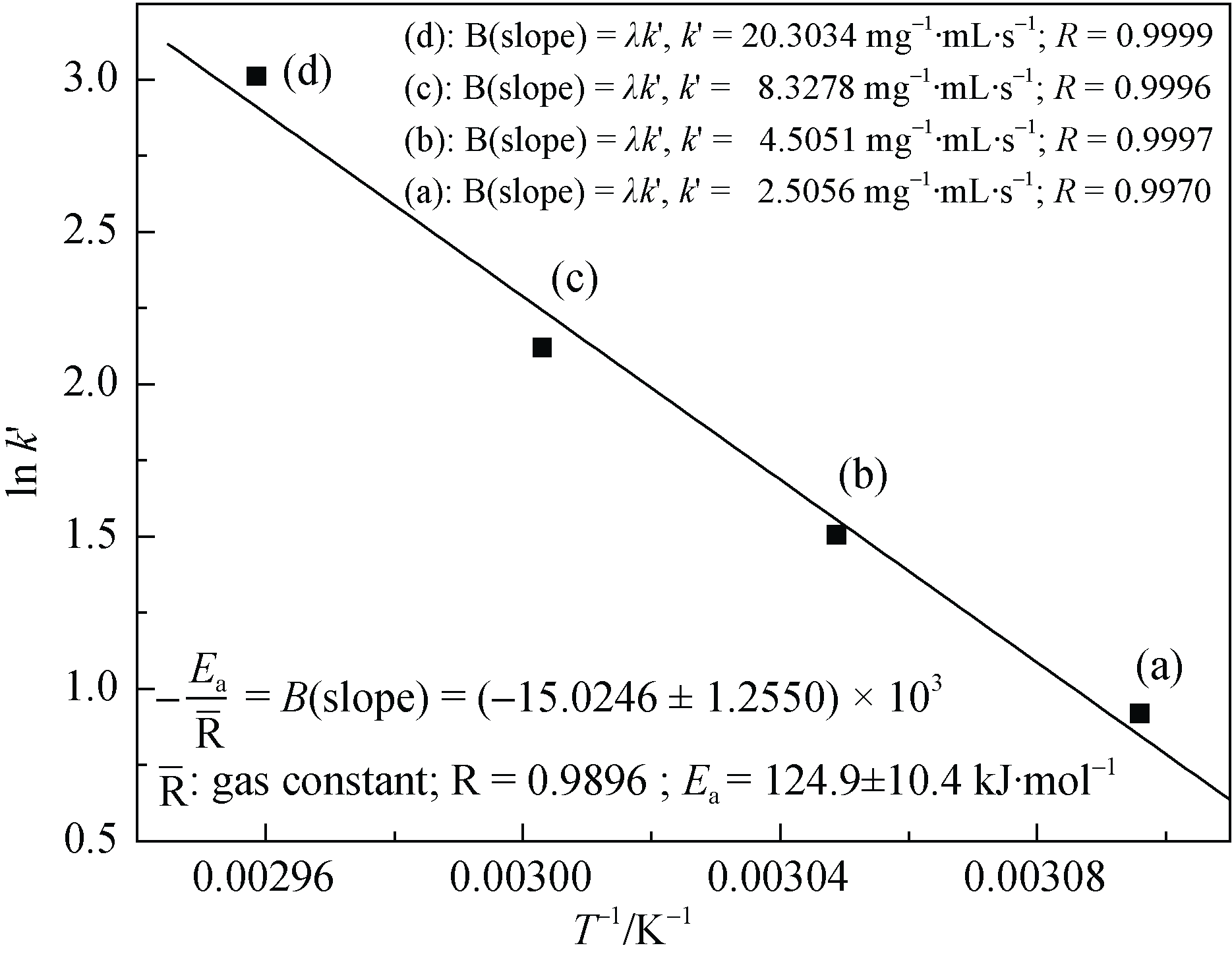
Fig. 5 Arrhenius plot of lnk° ~ 1 / T Reaction conditions: Ta=323 K, Tb=328 K, Tc=333 K, Td=338 K, TBHP (65 wt% in H2O) 1.00 mL, [Cu-buserite] 8.33 mg/mL, acetonitrile 5.00 mL
| Me-buserite | α | β | Ea /(kJ·mol-1) | k°/(mg-1·mL·s-1)* | V0.5 h /mL |
|---|---|---|---|---|---|
| Mg-buserite | 2 | 1 | 89 | 8.19 | 41.7 |
| Co-buserite | 2 | 1 | 56 | 2.38 | 20.0 |
| Ni-buserite | 2 | 1 | 96 | 5.66 | 33.6 |
| Cu-buserite | 2 | 1 | 125 | 20.30 | 55.6 |
Table 2 Kinetic parameters for disproportionation decomposition of TBHP catalyzed by Me-buserites and the O2 volume accumulated in a reaction period of 0.5 h
| Me-buserite | α | β | Ea /(kJ·mol-1) | k°/(mg-1·mL·s-1)* | V0.5 h /mL |
|---|---|---|---|---|---|
| Mg-buserite | 2 | 1 | 89 | 8.19 | 41.7 |
| Co-buserite | 2 | 1 | 56 | 2.38 | 20.0 |
| Ni-buserite | 2 | 1 | 96 | 5.66 | 33.6 |
| Cu-buserite | 2 | 1 | 125 | 20.30 | 55.6 |
| [1] | SCHWEGMAN J J, HARDWICK L M, AKERS M J.Practical formulation and process development of freeze-dried products.Pharmaceutical Development & Technology, 2005, 10(2): 151-173. |
| [2] | SALANITRO J P.Understanding the limitations of microbial metabolism of ethers used as fuel octane enhancers.Current Opinion in Biotechnology, 1995, 6(3): 337-340. |
| [3] | MENEZES E W D, CATALUNA R, SAMIOS D, et al. Addition of an azeotropic ETBE/ethanol mixture in euro super-type gasolines.Fuel, 2006, 85: 2567-2577. |
| [4] | SHI FENG, XIONG HAI, GU YAN-LONG, et al.The first non-acid catalytic synthesis of tert-butyl ether from tert-butyl alcohol using ionic liquid as dehydrator.Chemical Communications, 2003, 34(33): 1054-1055. |
| [5] | MIMOUN H, MIGNARD M, BRECHOT P, et al.Selective epoxidation of olefins by oxo[N-(2-oxidophenyl) salicylidenaminato] vanadium(V) alkylperoxides. On the mechanism of the Halcon epoxidation process.Journal of the American Chemical Society, 1986, 108(13): 3711-3718. |
| [6] | WINKLER D E, HEARNE G W.Liquid phase oxidation of isobutane.Industrial & Engineering Chemistry, 1961, 53(8): 655-658. |
| [7] | COYLE J J.Decomposition of Hydroperoxides in Propylene Epoxidation Reaction product. U.S. Patent NO. 4059598, 1977. |
| [8] | DIXIT P S, SRINIVASAN K.The effect of clay-support on the catalytic epoxidation activity of a manganese(III)-Schiff base complex.Inorganic Chemistry, 1988(24): 4507-4509. |
| [9] | RIAHI A, HENIN F, MUZART J.Homogeneous chromium(VI)-catalyzed oxidations of allylic alcohols by alkyl hydroperoxides: influence of the nature of the alkyl group on the product distribution.Tetrahedron Letters, 1999, 40(12): 2303-2306. |
| [10] | STOJANOVA M, KARSHALYKOV C, KANAZIREV V, et al.On the reactivity of H-, Ga- and Cu-MFI zeolites towards t-butyl hydroperoxide (TBHP).Applied Catalysis A General, 1996, 143(1): 175-183. |
| [11] | ROTHENBERG G, WIENER H, SASSON Y.Pyridines as bifunctional co-catalysts in the CrO3-catalyzed oxygenation of olefins by t-butyl hydroperoxide.Journal of Molecular Catalysis A Chemical, 1998, 136: 253-262. |
| [12] | HOUGHTON R P, RICE C R.Cobalt(II)-catalysed decomposition of hydroperoxides. Implications for alkane functionalization.Polyhedron, 1996, 15(11): 1893-1897. |
| [13] | TURRA N, NEUENSCHWANDER U, BAIKER A, et al.Mechanism of the catalytic deperoxidation of tert-butyl hydroperoxide with cobalt(II) acetylacetonate.Chemistry - A European Journal, 2010, 16(44): 13226-13235. |
| [14] | WEST Z J, ADAMS R K, ZZBARNICK S.Homogeneous catalysis of liquid-phase hydroperoxide decomposition in hydrocarbons.Energy Fuels, 2011, 25(3): 897-904. |
| [15] | YADAV G D, ASTHANA N S.Selective decomposition of cumene hydroperoxide into phenol and acetone by a novel cesium substituted heteropolyacid on clay.Applied Catalysis A General, 2003, 244(2): 341-357. |
| [16] | LUO Y, MAEDA S, OHNO K.Decomposition of alkyl hydroperoxide by a copper(I) complex: insights from density functional theory.Tetrahedron Letters, 2008, 49: 6841-6845. |
| [17] | RYAN P, KONSTANTINOV I, SNURR R Q, et al.DFT investigation of hydroperoxide decomposition over copper and cobalt sites within metal-organic frameworks.Journal of Catalysis, 2012, 286(4): 95-102. |
| [18] | KNIFTON J F.Method for One-step Synthesis of Methyl t-butyl ether. U.S. Patent NO. 4827048, 1989. |
| [19] | CUI H, LIU X, TAN W, et al.Influence of Mn(III) availability on the phase transformation from layered buserite to tunnel-structured todorokite.Clays & Clay Minerals, 2008, 56(4): 397-403. |
| [20] | WONG S T, CHENG S.Pillared layered manganese oxide synthesis and redox properties.Journal of Thermal Analysis, 1993, 40: 1181-1192. |
| [21] | WONG S T, CHENG S.Catalytic properties of layered and pillared buserites.Journal of the Chinese Chemical Society, 1993, 40: 509-516. |
| [22] | YANG MING, LING QIANG, YANG HONG-XIAO, et al.Enhanced catalytic activity of K-birnessite MnO2 confined in carbon nanotubes for selective oxidation of benzyl alcohol.Catalysis Communications, 2014, 46: 238-241. |
| [23] | WIECHEN M, ZAHARIEVA I, DAU H, et al.Layered manganese oxides for water-oxidation: alkaline earth cations influence catalytic activity in a photosystem II-like fashion.Chemical Science, 2012, 7(7): 2330-2339. |
| [24] | IYER A, DEL-PILAR J, KING’ONDU C K, et al. Water oxidation catalysis using amorphous manganese oxides, octahedral molecular sieves (OMS-2), and octahedral layered (OL-1) manganese oxide structures.The Journal of Physical Chemisry C, 2012, 116(10): 6474-6483. |
| [25] | ATRIBAK I, BUENO-LOPEZ A, GARCIA-GARCIA A, et al.Catalytic activity for soot combustion of birnessite and cryptomelane.Applied Catalysis B Environmental, 2010, 93(3/4): 267-273. |
| [26] | ZHANG XUAN-XUAN, RAN FEN, FAN HUI-LI, et al.Hydrothermal synthesis and electrochemical measurements of interconnected porous carbon/MnO2 composites.Acta Physico-Chimica Sinca, 2014, 30(5): 881-890. |
| [27] | SUN ZHENJIE, SHU DONG, CHEN HONGYU, et al.Microstructure and supercapacitive properties of buserite-type manganese oxide with a large basal spacing.Journal of Power Sources, 2012, 216(11): 425-433. |
| [28] | ATHOUEL L, MOSER F, DUGAS R, et al.Variation of the MnO2 birnessite structure upon charge/discharge in an electrochemical supercapacitor electrode in aqueous Na2SO4 electrolyte.Journal of Physical Chemistry C, 2008, 112(18): 7270-7277. |
| [29] | GHODBANE O, PASCAL J L, FAVIER F.Microstructural effects on charge-storage properties in MnO2-based electrochemical supercapacitors.Acs Applied materials & Interfaces, 2009, 1(5): 1130-1139. |
| [30] | RAMALINGAM K, KAMATCHI T, SUMOD P.Synthesis, spectral, thermal and CO2 absorption studies on birnessites type layered MnO6 oxide.Transition Metal Chemistry, 2006, 31(4): 429-433. |
| [31] | CHING S, KRUKOWSKA K S, SUIB S L.A new synthetic route to todorokite-type manganese oxides.Inorganica Chimica Acta, 1999, 294(2): 123-132. |
| [32] | MA Y, LUO J, SUIB S L.Syntheses of birnessites using alcohols as reducing reagents: effects of synthesis parameters on the formation of birnessites.Chemistry of Materials, 1999, 11(8): 1972-1979. |
| [33] | MAIR R D, GRAYPNER A J.Determination of organic peroxides by iodine liberation procedures.Analytical Chemistry, 1964, 36(1): 194-204. |
| [34] | LIU Y, TSUNOYAMA H, AKITA T, et al.Efficient and selective epoxidation of styrene with TBHP catalyzed by Au25 clusters on hydroxyapatite.Chemical Communications, 2010, 46(4): 550-552. |
| [1] | WANG Mengtao, SUO Jun, FANG Dong, Yi Jianhong, LIU Yichun, Olim RUZIMURADOVC. Visible-Light Catalytic Performance of ITO/TiO2 Nanotube Array Composite [J]. Journal of Inorganic Materials, 0, (): 178-. |
| [2] | SUN Chen, ZHAO Kunfeng, YI Zhiguo. Research Progress in Catalytic Total Oxidation of Methane [J]. Journal of Inorganic Materials, 0, (): 117-. |
| [3] | LI Yuejun, CAO Tieping, SUN Dawei. Bi4O5Br2/CeO2 Composite with S-scheme Heterojunction: Construction and Its CO2 Reduction Performance [J]. Journal of Inorganic Materials, 0, (): 3-. |
| [4] | TUERHONG Munire, ZHAO Honggang, MA Yuhua, QI Xianhui, LI Yuchen, YAN Chenxiang, LI Jiawen, CHEN Ping. Construction and Photocatalytic Activity of Monoclinic Tungsten Oxide/Red Phosphorus Step-scheme Heterojunction [J]. Journal of Inorganic Materials, 2023, 38(6): 701-707. |
| [5] | GAN Hongyu, FENG Yan, YANG Dehong, TIAN Yubin, LI Yang, XING Tao, LI Zhi, ZHAO Xuebo, DAI Pengcheng. Heteroatom-doped Biochar for Direct Dehydrogenation of Propane to Propylene [J]. Journal of Inorganic Materials, 2022, 37(10): 1058-1064. |
| [6] | MA Xinquan, LI Xibao, CHEN Zhi, FENG Zhijun, HUANG Juntong. BiOBr/ZnMoO4 Step-scheme Heterojunction: Construction and Photocatalytic Degradation Properties [J]. Journal of Inorganic Materials, 2023, 38(1): 62-70. |
| [7] | LIU Xuechen, ZENG Di, ZHOU Yuanyi, WANG Haipeng, ZHANG Ling, WANG Wenzhong. Selective Oxidation of Biomass over Modified Carbon Nitride Photocatalysts [J]. Journal of Inorganic Materials, 2022, 37(1): 38-44. |
| [8] | CHEN Xiaomei, CHEN Ying, YUAN Xia. Decomposition of Cyclohexyl Hydroperoxide Catalyzed by Core-shell Material Co3O4@SiO2 [J]. Journal of Inorganic Materials, 2022, 37(1): 65-71. |
| [9] | LI Tie, LI Yue, WANG Yingyi, ZHANG Ting. Preparation and Catalytic Properties of Graphene-Bismuth Ferrite Nanocrystal Nanocomposite [J]. Journal of Inorganic Materials, 2021, 36(7): 725-732. |
| [10] | FAN Jun, JIANG Xue, JIAO Yi, CHEN Yusheng, WANG Jianli, CHEN Yaoqiang. Effect of Different Alkali-assisted Deposition Precipitation Methods on the Durability of Three-way Catalysts [J]. Journal of Inorganic Materials, 2021, 36(6): 659-664. |
| [11] | AN Weijia, LI Jing, WANG Shuyao, HU Jinshan, LIN Zaiyuan, CUI Wenquan, LIU Li, XIE Jun, LIANG Yinghua. Fe(III)/rGO/Bi2MoO6 Composite Photocatalyst Preparation and Phenol Degradation by Photocatalytic Fenton Synergy [J]. Journal of Inorganic Materials, 2021, 36(6): 615-622. |
| [12] | XIAO Xiang, GUO Shaoke, DING Cheng, ZHANG Zhijie, HUANG Hairui, XU Jiayue. CsPbBr3@TiO2 Core-shell Structure Nanocomposite as Water Stable and Efficient Visible-light-driven Photocatalyst [J]. Journal of Inorganic Materials, 2021, 36(5): 507-512. |
| [13] | LIU Yaxin, WANG Min, SHEN Meng, WANG Qiang, ZHANG Lingxia. Bi-doped Ceria with Increased Oxygen Vacancy for Enhanced CO2 Photoreduction Performance [J]. Journal of Inorganic Materials, 2021, 36(1): 88-94. |
| [14] | WANG Juhan,WEN Xiong,LIU Chengchao,ZHANG Yuhua,ZHAO Yanxi,LI Jinlin. Preparation and Fischer-Tropsch Synthesis Performance of Hierarchical Co/Al-SiO2 Catalyst [J]. Journal of Inorganic Materials, 2020, 35(9): 999-1004. |
| [15] | DING Sheng, NING Kai, YUAN Binxia, PAN Weiguo, YIN Shibin, LIU Jianfeng. Durability of Fe-N/C Catalysts with Different Nanostructures for Electrochemical Oxygen Reduction in Alkaline Solution [J]. Journal of Inorganic Materials, 2020, 35(8): 953-958. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||