
Journal of Inorganic Materials ›› 2015, Vol. 30 ›› Issue (10): 1025-1030.DOI: 10.15541/jim20150091
• Orginal Article • Previous Articles Next Articles
GUO Dong-Xue1, ZHANG Qing-Hong1, WANG Hong-Zhi1, LI Yao-Gang1, CAO Guang-Xiu2
Received:2015-02-11
Revised:2015-04-24
Published:2015-10-20
Online:2015-09-30
About author:GUO Dong-Xue. E-mail:dxguo111@163.com
Supported by:CLC Number:
GUO Dong-Xue, ZHANG Qing-Hong, WANG Hong-Zhi, LI Yao-Gang, CAO Guang-Xiu. Preparation of RuO2/ZrO2/TaON Composite Photocatalyst and Its Photocatalytic Properties for Water Splitting Hydrogen Evolution[J]. Journal of Inorganic Materials, 2015, 30(10): 1025-1030.
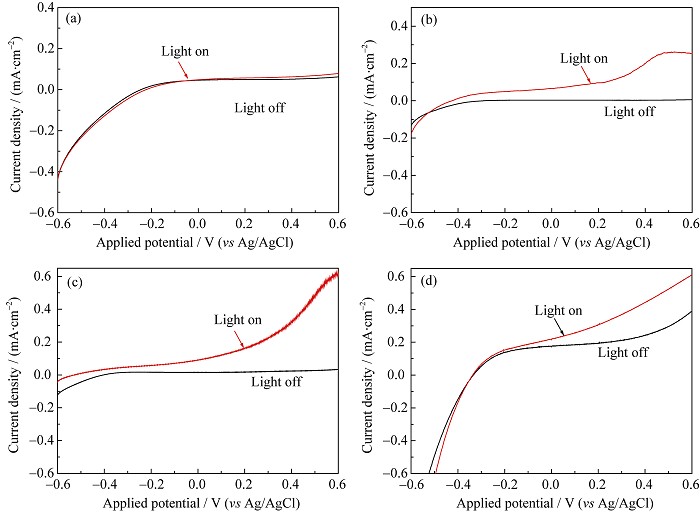
Fig. 6 Photocurrent densities under visible light irradiation of ZrO2/TaON (a) and RuO2/ZrO2/TaON photoanodes with different RuO2 loadings, 1.0wt% (b), 2.0wt% (c) and 3.0wt%(d)
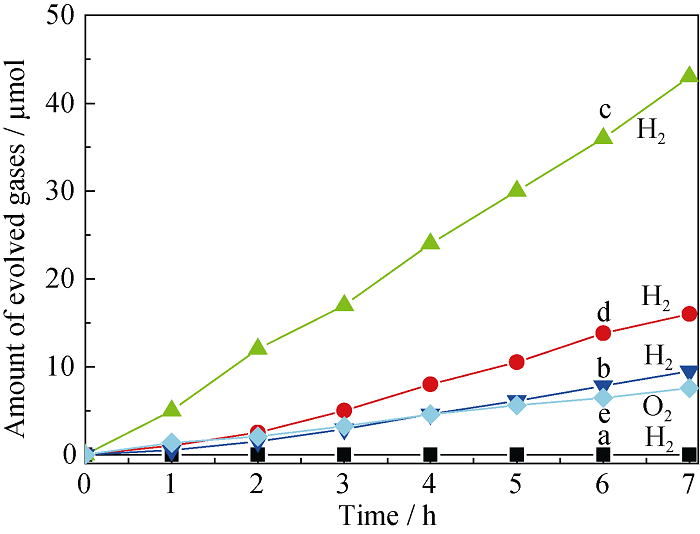
Fig. 7 Hydrogen production rates over photocatalysts ZrO2/ TaON (a) and RuO2/ZrO2/TaON with RuO2 loadings of 1.0wt% (b), 2.0wt% (c), 3.0wt% (d), and oxygen production rates over photocatalyst ZrO2/TaON with RuO2 loading of 3.0wt% (e)
| [1] | AHMAD H, KAMARUDIN S K, MINGGU L J, et al.Hydrogen from photo-catalytic water splitting process: a review.Renewable and Sustainable Energy Reviews, 2015, 43(1): 599-610. |
| [2] | ZHANG QING-HONG.Progress on TiO2-based nanomaterials and its utilization in the clean energy technology.Journal of Inorganic Materials, 2012, 27(1): 1-10. |
| [3] | TURNER J A.Sustainable hydrogen production.Science, 2004, 305(5686): 972-974. |
| [4] | CUI WEN-QUAN, LIU YAN-FEI, HU JIN-SHAN, et al.Synthesis of PbS intercalated K4Nb6O17 composite and its photocatalytic activity for hydrogen production.Journal of Inorganic Materials, 2012, 27(9): 933-938. |
| [5] | SANG HUAN-XIN, TIAN YE, WANG XI-TAO, et al.Photocatalytic H2 evolution from glycerol solution over Bi3+-doped TiO2 nanoparticles.Journal of Inorganic Materials, 2012, 27(12): 1283-1288. |
| [6] | MAEDA K, DOMEN K.New non-oxide photocatalysts designed for overall water splitting under visible light.The Journal of Physical Chemistry C, 2007, 111(22): 7851-7861. |
| [7] | JING D W, GUO L J, ZHAO L, et al.Efficient solar hydrogen production by photocatalytic water splitting: From fundamental study to pilot demonstration.International Journal of Hydrogen Energy, 2010, 35(13): 7087-7097. |
| [8] | KATO H, ASAKURA K, KUDOA. Highly efficient water splitting into H2 and O2 over lanthanum-doped NaTaO3 photocatalysts with high crystallinity and surface nanostructure.Journal of the American Chemical Society, 2003, 125(10): 3082-3089. |
| [9] | ZEUG N, BUCHELER J, KISCH H.Catalytic formation of hydrogen and carbon-carbon bonds on illuminated zinc sulfide generated from zinc dithiolenes.Journal of the American Chemical Society, 1985, 107(6): 1459-1465. |
| [10] | MAEDA K, TERAMURA K, LU D, et al.Photocatalyst releasing hydrogen from water.Nature, 2006, 440(7082): 295-301. |
| [11] | HITOKI G, TAKATA T, KONDO J.An oxynitride, TaON, as an efficient water oxidation photocatalyst under visible light irradiation (λ≤500 nm).Chemical Communications, 2002, 38(16): 1698-1699. |
| [12] | ABE R, HAGASHI M, DOEMN K.Facile fabrication of an efficient oxynitride TaON photoanode for over all water splitting into H2 and O2 under visible light irradiation.Journal of the American Chemical Society, 2010, 132(34):11828-11834. |
| [13] | EMMANUELLE O, FRANCK T, ROGER M.Synthesis and energetics of yellow TaON.Solid State Science, 2002, 4(8): 1071-1076. |
| [14] | MAEDA K, DOMEN K.Oxynitride materials for solar water splitting.MRS Bulletin, 2011, 36(1): 25-31. |
| [15] | CHUN W J, ISHIKAWA A, FUJISAWA H, et al.Conduction and valence band positions of Ta2O5, TaON, and Ta3N5 by UPS and electrochemical methods.The Journal of Physical Chemistry B, 2003, 107(8):1798-1803. |
| [16] | MAEDA K, TERASHIMA H, KASE K, et al.Nanoparticulate precursor route to fine particles of TaON and ZrO2-TaON solid solution and their photocatalytic activity hydrogen evolution under visible light.Applied Catalysis A: General, 2009, 357(2): 206-212. |
| [17] | MA S S K, MAEDA K, DOMEN K. Modification of TaON with ZrO2 to improve photocatalytic hydrogen evolution activity under visible light: influence of preparation conditions on activity.Catalysis Science & Technology, 2012, 2(4): 818-823. |
| [18] | RUI Y C, LI Y G, ZHANG Q H, et al.Size-tunable TiO2 nanorod microspheres synthesised via a one-pot solvothermal method and used as the scattering layer for dye-sensitized solar cells.Nanoscale, 2013, 5(24): 12574-12581. |
| [19] | ZHENG XIAN-JUN, JIANG QIAO-JUAN, WEI Li-Fang, et al.Preparation of nano- Eu/TiO2 and its photocatalytic production of H2 from formic acid solution.Chinese Rare Earths, 2010, 31(3): 58-61. |
| [20] | ZAHNG Q H, GAO L.Ta3N5 nanoparticles with enhanced photocatalytic efficiency under visible light irradiation.Langmuir, 2004, 20(22): 9821-9827. |
| [21] | MAEDA K, ABE R, DOMEN K.Role and function of ruthenium species as promoters with TaON-based photocatalysts for oxygen evolution in two-step water splitting under visible light.The Journal of Physical Chemistry C, 2011, 115(7): 3057-3064. |
| [22] | MAEDA K, LU D L, DOMEN K.Direct water splitting into hydrogen and oxygen under visible light by using modified TaON photocatalysts with d0 electronic configuration.Chemistry: A European Journal, 2013, 19(16): 4986-4991. |
| [23] | MAEDA K.Z-scheme water splitting using two different semiconductor photocatalysts.ACS Catalysis, 2013, 3(7): 1486-1503. |
| [24] | CHEN W, CHU M C, GAO L, et al.Ni(OH)2 loaded on TaON for enhancing photocatalytic water splittingactivity under visible light irradiation.Applied Surface Science, 2015, 324(1): 432-437. |
| [25] | ISMAIL A A, BAGNEMANN D W.Photochemical splitting of water for hydrogen production by photocatalysis: a review.Solar Energy Materials and Solar Cells, 2014, 128(1): 85-101. |
| [1] | TUERHONG Munire, ZHAO Honggang, MA Yuhua, QI Xianhui, LI Yuchen, YAN Chenxiang, LI Jiawen, CHEN Ping. Construction and Photocatalytic Activity of Monoclinic Tungsten Oxide/Red Phosphorus Step-scheme Heterojunction [J]. Journal of Inorganic Materials, 2023, 38(6): 701-707. |
| [2] | SUN Qiangqiang, CHEN Zixuan, YANG Ziyue, WANG Yimeng, CAO Baoyue. Amorphous Vanadium Oxide Loaded by Metallic Nickel-copper towards High-efficiency Electrocatalyzing Hydrogen Production [J]. Journal of Inorganic Materials, 2023, 38(6): 647-655. |
| [3] | WU Lin, HU Minglei, WANG Liping, HUANG Shaomeng, ZHOU Xiangyuan. Preparation of TiHAP@g-C3N4 Heterojunction and Photocatalytic Degradation of Methyl Orange [J]. Journal of Inorganic Materials, 2023, 38(5): 503-510. |
| [4] | MA Xinquan, LI Xibao, CHEN Zhi, FENG Zhijun, HUANG Juntong. BiOBr/ZnMoO4 Step-scheme Heterojunction: Construction and Photocatalytic Degradation Properties [J]. Journal of Inorganic Materials, 2023, 38(1): 62-70. |
| [5] | WANG Ruyi, XU Guoliang, YANG Lei, DENG Chonghai, CHU Delin, ZHANG Miao, SUN Zhaoqi. p-n Heterostructured BiVO4/g-C3N4 Photoanode: Construction and Its Photoelectrochemical Water Splitting Performance [J]. Journal of Inorganic Materials, 2023, 38(1): 87-96. |
| [6] | CHEN Hanxiang, ZHOU Min, MO Zhao, YI Jianjian, LI Huaming, XU Hui. 0D/2D CoN/g-C3N4 Composites: Structure and Photocatalytic Performance for Hydrogen Production [J]. Journal of Inorganic Materials, 2022, 37(9): 1001-1008. |
| [7] | HU Yue, AN Lin, HAN Xin, HOU Chengyi, WANG Hongzhi, LI Yaogang, ZHANG Qinghong. RhO2 Modified BiVO4 Thin Film Photoanodes: Preparation and Photoelectrocatalytic Water Splitting Performance [J]. Journal of Inorganic Materials, 2022, 37(8): 873-882. |
| [8] | XUE Hongyun, WANG Congyu, MAHMOOD Asad, YU Jiajun, WANG Yan, XIE Xiaofeng, SUN Jing. Two-dimensional g-C3N4 Compositing with Ag-TiO2 as Deactivation Resistant Photocatalyst for Degradation of Gaseous Acetaldehyde [J]. Journal of Inorganic Materials, 2022, 37(8): 865-872. |
| [9] | CHI Congcong, QU Panpan, REN Chaonan, XU Xin, BAI Feifei, ZHANG Danjie. Preparation of SiO2@Ag@SiO2@TiO2 Core-shell Structure and Its Photocatalytic Degradation Property [J]. Journal of Inorganic Materials, 2022, 37(7): 750-756. |
| [10] | WANG Xiaojun, XU Wen, LIU Runlu, PAN Hui, ZHU Shenmin. Preparation and Properties of Ag@C3N4 Photocatalyst Supported by Hydrogel [J]. Journal of Inorganic Materials, 2022, 37(7): 731-740. |
| [11] | AN Lin, WU Hao, HAN Xin, LI Yaogang, WANG Hongzhi, ZHANG Qinghong. Non-precious Metals Co5.47N/Nitrogen-doped rGO Co-catalyst Enhanced Photocatalytic Hydrogen Evolution Performance of TiO2 [J]. Journal of Inorganic Materials, 2022, 37(5): 534-540. |
| [12] | MA Hui, TAO Jianghui, WANG Yanni, HAN Yu, WANG Yabin, DING Xiuping. Gold Nanoparticles Supported on Silica & Titania Hybrid Mesoporous Spheres and Their Catalytic Performance Regulation [J]. Journal of Inorganic Materials, 2022, 37(4): 404-412. |
| [13] | ZHANG Xian, ZHANG Ce, JIANG Wenjun, FENG Deqiang, YAO Wei. Synthesis, Electronic Structure and Visible Light Photocatalytic Performance of Quaternary BiMnVO5 [J]. Journal of Inorganic Materials, 2022, 37(1): 58-64. |
| [14] | LIU Peng, WU Shimiao, WU Yunfeng, ZHANG Ning. Synthesis of Zn0.4(CuGa)0.3Ga2S4/CdS Photocatalyst for CO2 Reduction [J]. Journal of Inorganic Materials, 2022, 37(1): 15-21. |
| [15] | LIU Xuechen, ZENG Di, ZHOU Yuanyi, WANG Haipeng, ZHANG Ling, WANG Wenzhong. Selective Oxidation of Biomass over Modified Carbon Nitride Photocatalysts [J]. Journal of Inorganic Materials, 2022, 37(1): 38-44. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||