
Journal of Inorganic Materials ›› 2015, Vol. 30 ›› Issue (9): 913-918.DOI: 10.15541/jim20140671
• Orginal Article • Previous Articles Next Articles
MA Guo-Qiang, WEN Zhao-Yin, WANG Qing-Song, JIN Jun, WU Xiang-Wei, ZHANG Jing-Chao
Received:2014-12-25
Revised:2015-03-18
Published:2015-09-20
Online:2015-08-19
About author:MA Guo-Qiang. E-mail: guoqiangma@student.sic.ac.cn
Supported by:CLC Number:
MA Guo-Qiang, WEN Zhao-Yin, WANG Qing-Song, JIN Jun, WU Xiang-Wei, ZHANG Jing-Chao. Effects of CeO2 Nano-crystal on Electrochemical Properties of Lithium/Sulfur Batteries[J]. Journal of Inorganic Materials, 2015, 30(9): 913-918.
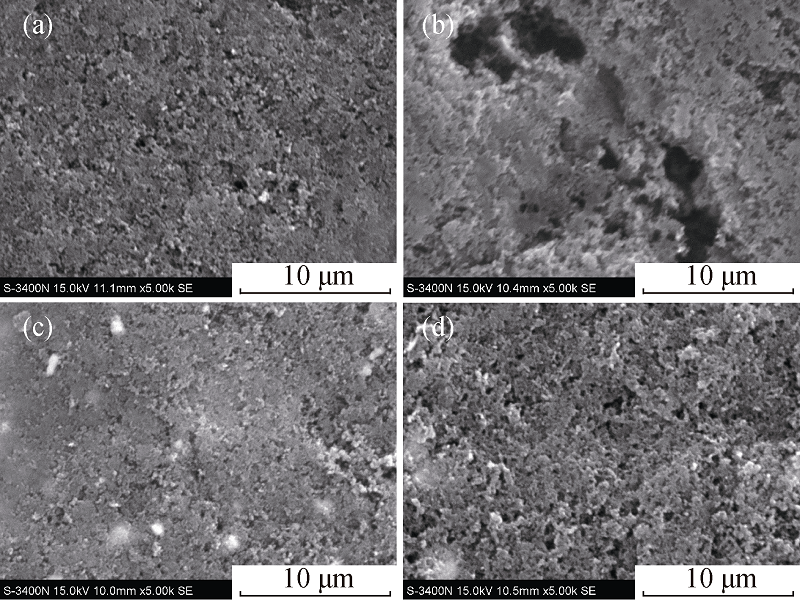
Fig. 4 SEM morphologies of sulfur electrodes (a) before cycling, (b) without CeO2 after 50 cycles, (c) with CeO2 before cycling and (d) with CeO2 after 50 cycles
| [1] | MANTHIRAM A, FU Y, SU Y S.Challenges and prospects of lithium/sulfur batteries.Accounts Chem. Res., 2012, 46(5): 1125-1134. |
| [2] | BRUCE P G, FREUNBERGER S A, HARDWICK L J, et al.Li-O2 and Li-S batteries with high energy storage.Nat. Mater., 2012, 11(1): 19-29. |
| [3] | ZHANG S S.Liquid electrolyte lithium/sulfur battery: fundamental chemistry, problems, and solutions.J. Power Sources, 2013, 231: 153-162. |
| [4] | KIM H, LIM H D, KIM J, et al.Graphene for advanced Li/S and Li/air batteries.Journal of Materials Chemistry A, 2014, 2: 33-47. |
| [5] | Evers S, Nazar L F.New approaches for high energy density lithium/ sulfur battery cathodes.Accounts Chem. Res., 2012, 46(5): 1135-1143. |
| [6] | SUO L, HU Y S, LI H, et al.A new class of solvent-in-salt electrolyte for high-energy rechargeable metallic lithium batteries.Nature Communications, 2013, 4: 1481-1489. |
| [7] | ZHENG J, GU M, WANG C, et al.Controlled nucleation and growth process of Li2S2/Li2S in lithium-sulfur batteries.Journal of the Electrochemical Society, 2013, 160: A1992-A1996. |
| [8] | ZHENG J, LV D, GU M, et al.How to obtain reproducible results for lithium sulfur batteries?Journal of the Electrochemical Society, 2013, 160: A2288-A2292. |
| [9] | YANG Y, ZHENG G, CUI Y.Nanostructured sulfur cathodes.Chem. Soc. Rev., 2013, 42: 3018-3032. |
| [10] | FU Y, SU Y S, MANTHIRAM A.Sulfur-carbon nanocomposite cathodes improved by an amphiphilic block copolymer for high-rate lithium-sulfur batteries.Acs. Appl. Mater. Inter., 2012, 4: 6046-6052. |
| [11] | ZHAO C, LIU L, ZHAO H, et al.Sulfur-infiltrated porous carbon microspheres with controllable multi-modal pore size distribution for high energy lithium-sulfur batteries.Nanoscale, 2014, 6(2): 882-888. |
| [12] | YANG Y, YU G, CHA J J, et al.Improving the performance of lithium-sulfur batteries by conductive polymer coating.Acs Nano, 2011, 5: 9187-9193. |
| [13] | LIANG X, LIU Y, WEN Z, et al.A nano-structured and highly ordered polypyrrole-sulfur cathode for lithium-sulfur batteries.J. Power Sources, 2011, 196: 6951-6955. |
| [14] | SU Y S, MANTHIRAM A.A new approach to improve cycle performance of rechargeable lithium-sulfur batteries by inserting a free-standing MWCNT interlayer.Chem. Commun., 2012, 48: 8817-8819. |
| [15] | CHEN J J, JIA X, SHE Q J, et al.The preparation of nano-sulfur/ MWCNTs and its electrochemical performance.Electrochim Acta, 2010, 55: 8062-8066. |
| [16] | YIN L C, WANG J L, YANG J, et al.A novel pyrolyzed polyacrylonitrile-sulfur@MWCNT composite cathode material for high-rate rechargeable lithium/sulfur batteries.J. Mater. Chem., 2011, 21: 6807-6810. |
| [17] | ZHENG G, ZHANG Q, CHA J J, et al.Amphiphilic surface modification of hollow carbon nanofibers for improved cycle life of lithium sulfur batteries.Nano Lett., 2013, 13(3): 1265-1270. |
| [18] | JAYAPRAKASH N, SHEN J, MOGANTY S S, et al.A. porous hollow carbon@sulfur composites for high-power lithium-sulfur batteries.Angewandte Chemie International Edition, 2011, 123(26): 6026-6030. |
| [19] | ZHANG K, ZHAO Q, TAO Z, et al.Composite of sulfur impregnated in porous hollow carbon spheres as the cathode of Li-S batteries with high performance.Nano Res., 2012, 6: 38-46. |
| [20] | SEH Z W, LI W, CHA J J, et al.Sulphur-TiO2 yolk-shell nanoarchitecture with internal void space for long-cycle lithium- sulphur batteries.Nature Communications, 2013, 4: 1331-1336. |
| [21] | LI J, DING B, XU G, et al.Enhanced cycling performance and electrochemical reversibility of a novel sulfur-impregnated mesoporous hollow TiO2 sphere cathode for advanced Li-S batteries.Nanoscale, 2013, 5(13): 5743-5746. |
| [22] | ZHANG Y, ZHAO Y, KONAROV A, et al.One-pot approach to synthesize PPy@S core-shell nanocomposite cathode for Li/S batteries.J. Nanopart Res., 2013, 15(10): 1-7. |
| [23] | ZHOU W, YU Y, CHEN H, et al.Yolk-shell structure of polyaniline coated sulfur for lithium-sulfur batteries.J. Am. Chem. Soc., 2013, 135(44): 16736-16743. |
| [24] | XIAO L, CAO Y, XIAO J, et al.A soft approach to encapsulate sulfur: polyaniline nanotubes for lithium-sulfur batteries with long cycle life.Adv. Mater., 2012, 24: 1176-1181. |
| [25] | WANG C, WAN W, CHEN J T, et al.Dual core-shell structured sulfur cathode composite synthesized by a one-pot route for lithium sulfur batteries. Journal of Materials Chemistry A, 2013, 1: 1716-1723. |
| [26] | ZHANG Y, WANG L, ZHANG A, et al.Novel V2O5/S composite cathode material for the advanced secondary lithium batteries.Solid State Ionics, 2010, 181: 835-838. |
| [27] | CHOI Y J, JUNG B S, LEE D J, et al.Electrochemical properties of sulfur electrode containing nano Al2O3 for lithium/sulfur cell.Phys Scripta, 2007, T129: 62-65. |
| [28] | SONG M S, HAN S C, KIM H S, et al.Effects of nanosized adsorbing material on electrochemical properties of sulfur cathodes for Li/S secondary batteries.Journal of the Electrochemical Society, 2004, 151: A791-A795. |
| [29] | ZHANG Y, WU X, FENG H, et al.Effect of nanosized Mg0.8Cu0.2O on electrochemical properties of Li/S rechargeable batteries.Int. J. Hydrogen. Energ., 2009, 34: 1556-1559. |
| [30] | XU R, BELHAROUAK I, ZHANG X, et al.New developments in lithium sulfur batteries[C]//SPIE defense, security, and sensing. International Society for Optics and Photonics, 2013, 10: 872804. |
| [31] | EVERS S, YIM T, NAZAR L F.Understanding the nature of absorption/adsorption in nanoporous polysulfide sorbents for the Li-S battery.The Journal of Physical Chemistry C, 2012, 116: 19653-19658. |
| [32] | LIANG X, WEN Z, LIU Y, et al.A composite of sulfur and polypyrrole-multi walled carbon combinatorial nanotube as cathode for Li/S battery.J. Power. Sources, 2012, 206: 409-413. |
| [33] | LI G C, HU J J, LI G R, et al.Sulfur/activated-conductive carbon black composites as cathode materials for lithium/sulfur battery.J. Power Sources, 2013, 240: 598-605. |
| [34] | ZHANG B, LAI C, ZHOU Z, et al.Preparation and electrochemical properties of sulfur-acetylene black composites as cathode materials.Electrochim Acta, 2009, 54: 3708-3713. |
| [35] | ZHOU G, PEI S, LI L, et al.A graphene-pure-sulfur sandwich structure for ultrafast, long-life lithium-sulfur batteries.Adv. Mater., 2014, 26(4): 625-631. |
| [1] | YUAN Jingkun, XIONG Shufeng, CHEN Zhangwei. Research Trends and Challenges of Additive Manufacturing of Polymer-derived Ceramics [J]. Journal of Inorganic Materials, 2023, 38(5): 477-488. |
| [2] | FU Shi, YANG Zengchao, LI Honghua, WANG Liang, LI Jiangtao. Mechanical Properties and Thermal Conductivity of Si3N4 Ceramics with Composite Sintering Additives [J]. Journal of Inorganic Materials, 2022, 37(9): 947-953. |
| [3] | JIANG Yiyi, SHEN Min, SONG Banxia, LI Nan, DING Xianghuan, GUO Leyi, MA Guoqiang. Effect of Dual-functional Electrolyte Additive on High Temperature and High Voltage Performance of Li-ion Battery [J]. Journal of Inorganic Materials, 2022, 37(7): 710-716. |
| [4] | NAN Bo, ZANG Jiadong, LU Wenlong, YANG Tingwang, ZHANG Shengwei, ZHANG Haibo. Recent Progress on Additive Manufacturing of Piezoelectric Ceramics [J]. Journal of Inorganic Materials, 2022, 37(6): 585-595. |
| [5] | CAO Jiwei, WANG Pei, LIU Zhiyuan, LIU Changyong, WU Jiamin, CHEN Zhangwei. Research Progress on Powder-based Laser Additive Manufacturing Technology of Ceramics [J]. Journal of Inorganic Materials, 2022, 37(3): 241-254. |
| [6] | LIU Haifang, SU Haijun, SHEN Zhonglin, JIANG Hao, ZHAO Di, LIU Yuan, ZHANG Jun, LIU Lin, FU Hengzhi. Research Progress on Ultrahigh Temperature Oxide Eutectic Ceramics by Laser Additive Manufacturing [J]. Journal of Inorganic Materials, 2022, 37(3): 255-266. |
| [7] | LIU Kai, SUN Ce, SHI Yusheng, HU Jiaming, ZHANG Qingqing, SUN Yunfei, ZHANG Song, TU Rong, YAN Chunze, CHEN Zhangwei, HUANG Shangyu, SUN Huajun. Current Status and Prospect of Additive Manufacturing Piezoceramics [J]. Journal of Inorganic Materials, 2022, 37(3): 278-288. |
| [8] | LIU Guoqian, YAN Changhai, ZHANG Keqiang, JIN Hua, HE Rujie. Effect of Solid Loading on the Property of Al2O3 Ceramics in Stereolithographic Additive Manufacturing [J]. Journal of Inorganic Materials, 2022, 37(3): 353-360. |
| [9] | LI Gaoran, LI Hongyang, ZENG Haibo. Recent Progress of Boron-based Materials in Lithium-sulfur Battery [J]. Journal of Inorganic Materials, 2022, 37(2): 152-162. |
| [10] | LI Tingting, ZHANG Yang, CHEN Jiahang, MIN Yulin, WANG Jiulin. Flexible Binder for S@pPAN Cathode of Lithium Sulfur Battery [J]. Journal of Inorganic Materials, 2022, 37(2): 182-188. |
| [11] | FU Yukun, ZENG Min, RAO Xianfa, ZHONG Shengwen, ZHANG Huijuan, YAO Wenli. Microwave-assisted Synthesis and Co, Al Co-modification of Ni-rich LiNi0.8Mn0.2O2 Materials for Li-ion Battery Electrode [J]. Journal of Inorganic Materials, 2021, 36(7): 718-724. |
| [12] | LIU Wenwen, HU Zhilei, WANG Li, CAO Mengsha, ZHANG Jing, ZHANG Jing, ZHANG Shuai, YUAN Ningyi, DING Jianning. Passiviation of L-3-(4-Pyridyl)-alanine on Interfacial Defects of Perovskite Solar Cell [J]. Journal of Inorganic Materials, 2021, 36(6): 629-636. |
| [13] | ZHU Quan, HU Jianbao, YANG Jinshan, ZHOU Haijun, DONG Shaoming. Strong SiC Porous Ceramic Obtained by Sintering of Reticulated Aligned SiC Nanowires [J]. Journal of Inorganic Materials, 2021, 36(5): 547-551. |
| [14] | JIANG Hao,WU Hao,HOU Chengyi,LI Yaogang,XIAO Ru,ZHANG Qinghong,WANG Hongzhi. Sawing Angles on Property of Lithium-sulfur Battery Interlayer Prepared with Birch Derived Orientedly Microchannel Biochar [J]. Journal of Inorganic Materials, 2020, 35(6): 717-723. |
| [15] | ZHANG Xiao-Feng,ZHANG Guan-Hua,MENG Yue,XUE Ji-Long,XIA Sheng-Jie,NI Zhe-Ming. Photocatalytic Degradation of Methylene Blue by Schiff-base Cobalt Modified CoCr Layered Double Hydroxides [J]. Journal of Inorganic Materials, 2019, 34(9): 974-982. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||