
Journal of Inorganic Materials ›› 2015, Vol. 30 ›› Issue (1): 77-80.DOI: 10.15541/jim20140259
• Orginal Article • Previous Articles Next Articles
MA Xi-Fei, KANG Zhuang, HUANG Xiao, ZHANG Guo-Jun
Received:2014-05-19
Revised:2014-06-23
Published:2015-01-20
Online:2014-12-29
About author:MA Xi-Fei. E-mail: maxifei@126.com
Supported by:CLC Number:
MA Xi-Fei, KANG Zhuang, HUANG Xiao, ZHANG Guo-Jun. Synthesis of ZrN Nanopowder via Soft Urea Pathway[J]. Journal of Inorganic Materials, 2015, 30(1): 77-80.
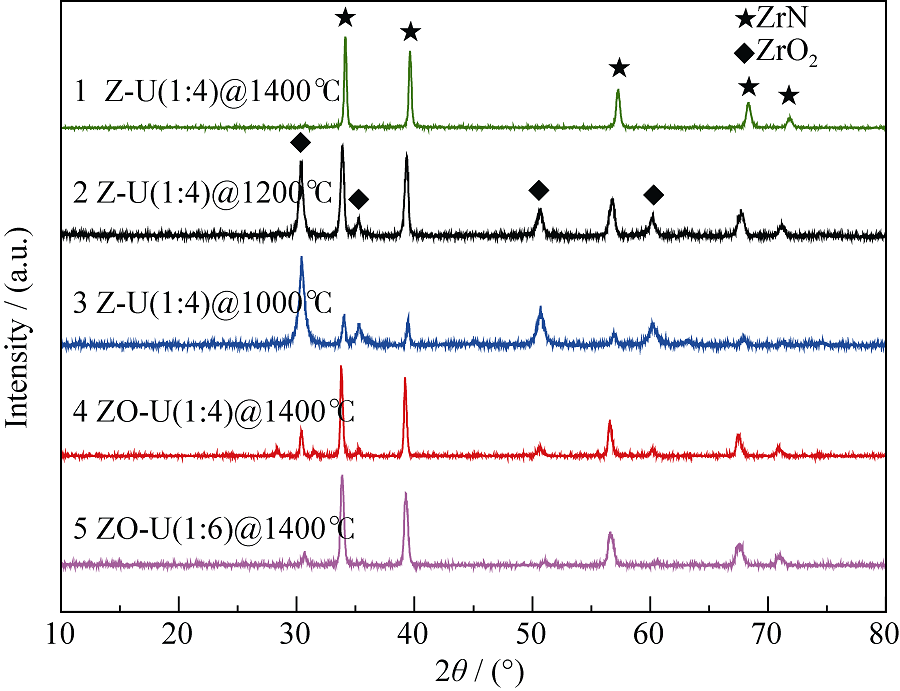
Fig. 4 XRD patterns of the products from different precursors pyrolyzed at different temperatures. From top to bottom: products of Z-U pyrolyzed at 1400℃, 1200℃, 1000℃ and ZO-U pyrolyzed at 1400℃ in N2 for 2 h, respectively
| [1] | SUN F H, ZHANG Z M, CHEN M, et al.Improvement of adhesive strength and surface roughness of diamond films on Co-cemented tungsten carbide tools.Diam. Relat. Mater., 2003, 12(3/7): 711-718. |
| [2] | ADACHI J, KUROSAKI K.Thermal and electrical properties of zirconium nitride. J. Alloys Compd., 2005, 399(1/2): 242-244. |
| [3] | TANG Y, ZHANG G J, XUE J X, et al.Densification and mechanical properties of hot-pressed ZrN ceramics doped with Zr or Ti.J. Eur. Ceram. Soc., 2013, 33(7): 1363-1371. |
| [4] | STREIT M, INGOLD F, POUCHON M, et al.Zirconium nitride as inert matrix for fast systems. J. Nucl. Mater., 2003, 319(1): 51-58. |
| [5] | ZHAO X J, CHENA D L, RUB H Q, et al.Zirconium nitride nano-particulate reinforced Alon composites: fabrication, mechanical properties and toughening mechanisms.J. Eur. Ceram. Soc., 2011, 31(5): 883-892. |
| [6] | ZHANG N, ZHAO X J.Thermal shock behavior of nano-sized ZrN particulate reinforced AlON composites.Ceram. Int., 2013, 39(1): 367-375. |
| [7] | ANDRIEVSKI R A.Nanomaterials based on high-melting carbides, nitrides and borides.Usp. Khim, 2005, 74(12): 1163-1175. |
| [8] | OYAMA S T, YU C C, RAMANTHAN S.Transition metal bimetallic oxycarbides: synthesis, characterization, and activity studies.J. Catal., 1999, 184(2): 535-549. |
| [9] | WU D, ZHANG Z, FU W, et al.Structure, electrical and chemical properties of zirconium nitride films deposited by dc reactive magnetron sputtering.Appl. Phys. A, 1997, 64(6): 593-595. |
| [10] | ALEXANDER A M, HARGREAVES J S J. Alternative catalytic materials: carbides, nitrides, phosphides and amorphous boron alloys. Chem. Soc. Rev., 2010, 39(11): 4388-4401. |
| [11] | CHEN H Y, WANG L P, BAI J M, et al.In situ XRD studies of ZnO/GaN mixtures at high pressure and high temperature: synthesis of Zn-Rich (Ga1-xZnx)(N1-xOx) photocatalysts.J. Phys. Chem. C, 2010, 114(4): 1809-1814. |
| [12] | SCHAAF P, KAHLE M, CARPENE E.Reactive laser synthesis of carbides and nitrides. Appl. Surf. Sci., 2005, 247(1-4): 607-615. |
| [13] | VISSOKOV G, TSVATANOV T.On the plasma-chemical synthesis of nanopowders.Plasma Sci. Technol., 2003, 5(6): 2039-2050. |
| [14] | FU B, GAO L.La1+xSr1-xGa3O7-δ Melilite-type ceramics-preparation, composition, and structure,J. Am. Ceram. Soc., 2004, 8(4): 696-699. |
| [15] | CHAU L H K, KAO C C. Microwave plasma synthesis of TiN and ZrN nanopowders.J. Mater. Lett., 2007, 61(14): 1583-1587. |
| [16] | SHEERIF E, ESKANADARANY M, ASHOUR A H.Mechanically induced gas-solid reaction for the synthesis of nanocrystalline ZrN powders and their subsequent consolidations. J. Alloys Compd., 2000, 313(1/2): 224-234. |
| [17] | YI H C, MOORE J J.Review self-propagating high-temperature (combustion) synthesis (SHS) of powder- compacted materials. J. Mater. Sci. Technol., 1990, 25(2): 1159-1168. |
| [18] | REDDY R S, KAMARAJ M.Generation and characterization of zirconium nitride nanoparticlesby wire explosion process. Ceram. Int., 2012, 38(7): 5507-5512 |
| [19] | YAMAMURA H, EMOTO S.Factors affecting the formation rate of ZrN by the carbothermal nitridation method.Nippon Seramikkusu Kyokai Gakujutsu Ronbunshi, 1998, 106(7): 650-653. |
| [20] | ORHAN E F, TESSIER F, MARCHAND R.Synthesis and energetics of yellow TaON.Solid State Sci., 2002, 4(8): 1071-1076. |
| [21] | PODSIADLO S.Stages of the synthesis of gallium nitride with the use of urea.Thermochim. Acta, 1995, 256(2): 367-373. |
| [22] | GIORDANO C, ANTONIRTTI M.Synthesis of crystalline metal nitride and metal carbide nanostructures by Sol-Gel chemistry.Nano Today, 2011, 6(4): 366-380. |
| [23] | YAO W, MAKOWSKI P, GIORDANO C, et al.Synthesis of early transition metal carbide and nitride nanoparticles through the urea route and their use as alkylation catalysts. J. Chem. Eur., 2009, 15(44): 11999-12004. |
| [24] | WADE G Jr. Organic Chemistry, 4th Ed. Prentice Hall, New Jersey, 1979. |
| [25] | SRIVASTAVA A.Low-temperature preparation of tetragonal zirconia.Mater. Lett., 1987, 5(3): 111-115. |
| [26] | STEWART J E.Infrared absorption spectra of urea, thiourea, and some thiourea-alkali halide complexes.J. Chem. Phys., 1957, 26(2): 248-254. |
| [27] | SANTOS V, ZENI M, BERGMANN C P.Correlation between thermal treatment and tetragonal/monoclinic nanostructured zirconia powder obtained by Sol-Gel process.Rev. Adv. Mater. Sci., 2008, 17(1/2): 62-70. |
| [28] | QIU Y, GAO L.Synthesis of nanocrystalline CrN from Cr[OC(NH2)2]6Cl3 coordination compound. Mater. Res. Bull, 2003, 38(9): 1551-1557. |
| [29] | ZAHARESCU M, JITIANU A.Composition and thermal stability of SiO2-based hybrid materials TEOS-MTEOS system.Therm. Anal. Calorim., 2003, 71(2): 421-428. |
| [30] | YANG H Z, SHI T J, ZHAI L F, et al.Electrostatic spinnability of silica sol and morphologies of its electrospun product.J. Inorg. Chem., 2006, 21(5): 1273-1277. |
| [1] | WU Junlin, DING Jiyang, HUANG Xinyou, ZHU Danyang, HUANG Dong, DAI Zhengfa, YANG Wenqin, JIANG Xingfen, ZHOU Jianrong, SUN Zhijia, LI Jiang. Fabrication and Microstructure of Gd2O2S:Tb Scintillation Ceramics from Water-bath Synthesized Nano-powders: Influence of H2SO4/Gd2O3 Molar Ratio [J]. Journal of Inorganic Materials, 2023, 38(4): 452-460. |
| [2] | YANG Ying, ZHANG Zheng, GAO Jing, LIN Ze-Hua, YAN Jing-Yuan, GUO Xue-Yi. Deep Eutectic Solvent Based Polymer Electrolyte for Dye-sensitized Solar Cells [J]. Journal of Inorganic Materials, 2017, 32(1): 25-32. |
| [3] | ZHAO Qian, WU Ping. Ferromagnetism Induced by Defects in Cr-doped TiO2 Nanopowders [J]. Journal of Inorganic Materials, 2013, 28(10): 1098-1102. |
| [4] | SU Xing-Hua, LI Jian-Gong, ZHOU Zhen-Jun. Solid-state Reaction Preparation and Sintering Behavior of MgAl2O4 Nanopowders [J]. Journal of Inorganic Materials, 2012, 27(9): 991-996. |
| [5] | WU Yi-Qing, NI Jian-Sen, DU Ya-Nan, HU Peng-Fei, DING Wei-Zhong, GENG Shu-Hua. Preparation of CeO2 Nanopowders by Hydrolysis and Oxidation of Cerium Carbide [J]. Journal of Inorganic Materials, 2012, 27(5): 489-494. |
| [6] | SONG Wei, WANG Xuan, ZHANG Dong, SUN Zhi, HAN Bai, HE Li-Juan, LEI Qing-Quan. Preparation and Characterization of Multiferroics BiFeO3 [J]. Journal of Inorganic Materials, 2012, 27(10): 1053-1057. |
| [7] | GAO Hong-Quan, WANG Xin-Yu, ZHANG Zhi-An, LAI Yan-Qing, LI Jie, LIU Ye-Xiang. Modification of Li4Ti5O12 Anode Material with Urea as Nitrogen Source for Lithium Ion Battery [J]. Journal of Inorganic Materials, 2010, 25(9): 983-988. |
| [8] | YU Cui, ZHU Tie-Jun, XIAO Kai, JIN Ji, SHEN Jun-Jie, YANG Sheng-Hui, ZHAO Xin-Bing. Microstructure of ZrNiSn-base Half-Heusler Thermoelectric Materials Prepared by Melt-spinning [J]. Journal of Inorganic Materials, 2010, 25(6): 569-572. |
| [9] | LI Xiao-Guang, HUO De-Xuan, HE Cai-Jun, ZHAO Shi-Chao, Lü Yan-Fei. Effect of Rare-earth Doping on the Thermoelectric Properties of the Tin-based Half-Heusler Alloys [J]. Journal of Inorganic Materials, 2010, 25(6): 573-576. |
| [10] | JIANG Dong-Liang. Transparent Ceramics:One of the Most Important Field of Research and Development of Inorganic Materials [J]. Journal of Inorganic Materials, 2009, 24(5): 873-881. |
| [11] | LI Xiu-Yan,YANG Xian-Feng,WU Ming-Mei. Photocatalytic Activities of TiO2 Nanopowders by Hydrothermal Synthesis in Different Solution Medium [J]. Journal of Inorganic Materials, 2008, 23(6): 1253-1258. |
| [12] | LIU Wen-Bin,KOU Hua-Min,PAN Yu-Bai,KARAKI Tomoaki,LI Jiang,GUO Jing-Kun. Preparation and Characteristic of Properties of High Transmission Nd:YAG Transparent Ceramic [J]. Journal of Inorganic Materials, 2008, 23(5): 1037-1040. |
| [13] | AN Li-Qiong,ZHANG Jian,LIU Min,WANG Shi-Wei. Spectroscopic Study of Lu2O3:Yb3+, Ho3+ Nanopowders [J]. Journal of Inorganic Materials, 2008, 23(2): 383-386. |
| [14] | ZHONG Zhi-Cheng,ZHANG Duan-Ming,HAN Xiang-yun,WEI Nian,YANG Feng-Xia,ZHENG Ke-Yu. Hydrothermal and Solvothermal Preparation of Nanocrystalline KTN Powders [J]. Journal of Inorganic Materials, 2007, 22(1): 45-48. |
| [15] | WEN Lei,SUN Xu-Dong,QI Lu,XU Guo-Xiang. Solution-based Processing of Y2O3 Nanopowders Yielding Transparent Ceramics [J]. Journal of Inorganic Materials, 2006, 21(3): 539-546. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||