
Journal of Inorganic Materials ›› 2014, Vol. 29 ›› Issue (12): 1287-1293.DOI: 10.15541/jim20140192
• Orginal Article • Previous Articles Next Articles
CHEN Shu-Qing1, 2, LÜ Gong-Xuan1
Received:2014-04-11
Revised:2014-06-24
Published:2014-12-20
Online:2014-11-20
About author:CHEN Shu-Qing. E-mail: chenshuqing139@163.com
Supported by:CLC Number:
CHEN Shu-Qing, Lü Gong-Xuan. CO2 Methanation over Ru/TiO2 Catalysts under UV Irradiation and Heating[J]. Journal of Inorganic Materials, 2014, 29(12): 1287-1293.
| Catalyst | SBET /(m2·g-1) | Vpore /(cm3·g-1) | Dpore /nm |
|---|---|---|---|
| P25 | 50.5 | 0.54 | 44.4 |
| 0.5%Ru/P25 | 48.3 | 0.26 | 21.5 |
| 1.0%Ru/P25 | 45.7 | 0.25 | 21.9 |
| 1.5%Ru/P25 | 49.7 | 0.28 | 22.6 |
| 2.0%Ru/P25 | 46.6 | 0.24 | 21.0 |
| 1.5%Ru/P25 (used)a | 46.8 | 0.23 | 21.2 |
Table 1 Physicochemical properties of the support and Ru/P25 catalysts
| Catalyst | SBET /(m2·g-1) | Vpore /(cm3·g-1) | Dpore /nm |
|---|---|---|---|
| P25 | 50.5 | 0.54 | 44.4 |
| 0.5%Ru/P25 | 48.3 | 0.26 | 21.5 |
| 1.0%Ru/P25 | 45.7 | 0.25 | 21.9 |
| 1.5%Ru/P25 | 49.7 | 0.28 | 22.6 |
| 2.0%Ru/P25 | 46.6 | 0.24 | 21.0 |
| 1.5%Ru/P25 (used)a | 46.8 | 0.23 | 21.2 |
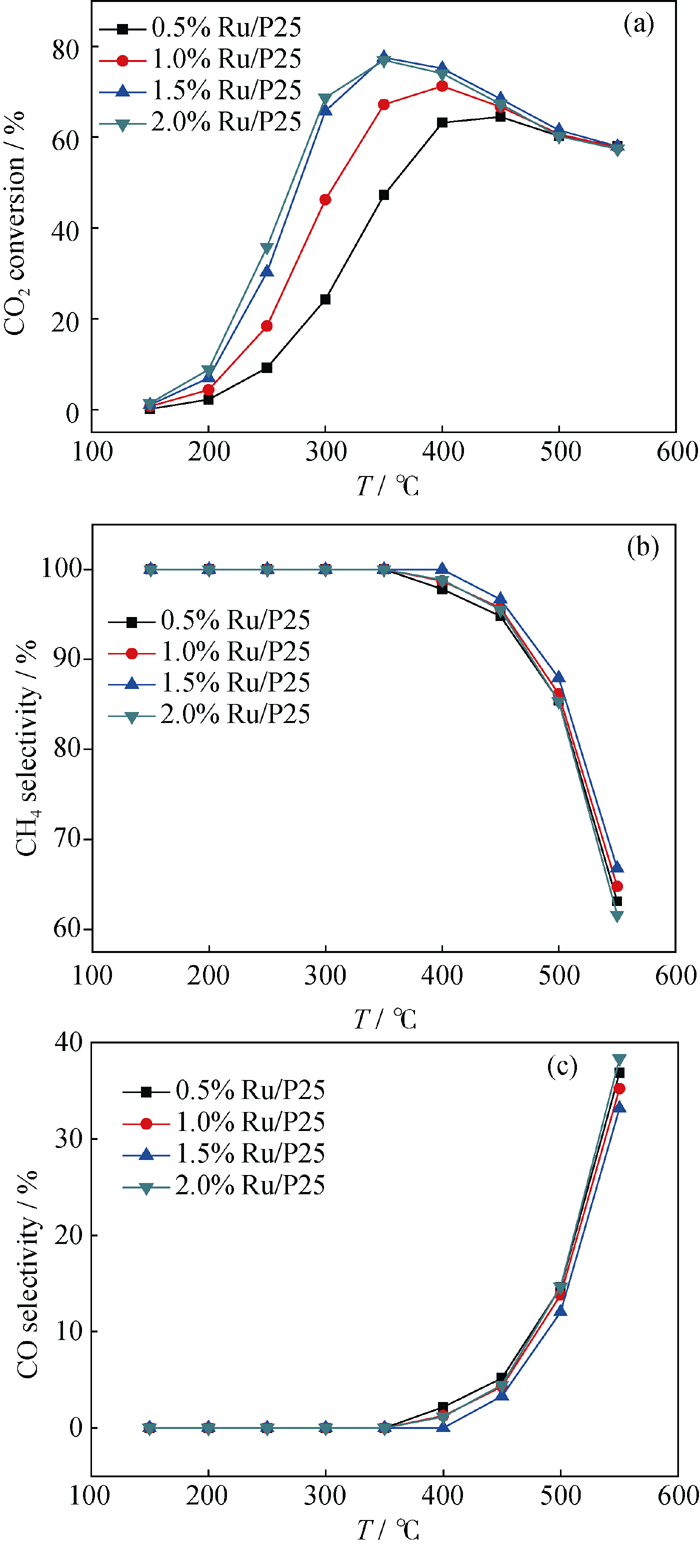
Fig. 5 Conversion of CO2 (a), CH4 selectivity (b) and CO sele-c-tivity (c) over the 0.5%-2.0% Ru/P25 catalyst at different temperatures Reaction conditions: 0.3 g catalyst; H2: CO2=4: 1; T=350℃, P = 105 Pa
| [1] | MIKKELSEN M, JORGENSEN M, KREBS F C.The teraton challenge. a review of fixation and transformation of carbon dioxide. Energy Environ. Sci., 2010, 3: 43-81. |
| [2] | WANG W, WANG S P, MA X B, et al.Recent advances in catalytic hydrogenation of carbon dioxide. Chem. Soc. Rev., 2011, 40(7): 3703-3727. |
| [3] | DING M Y, YANG Y, WU B S, et al.Study of phase transformation and catalytic performance on precipitated iron-based catalyst for Fischer-Tropsch synthesis,J. Mol. Catal. A: Chem., 2009, 303: 65-71. |
| [4] | KHODAKOV A Y, WEI C, PASCAL F.Advances in the development of novel cobalt Fischer-Tropsch catalysts for synthesis of long-chain hydrocarbons and clean fuels. Chem. Rev., 2007, 107: 1692-1744. |
| [5] | BORG Ø, ERI S, BLEKKAN E A, et al. Fischer-Tropsch synthesis over γ-alumina-supported cobalt catalysts: Effect of support variables. J. Catal., 2007, 248: 89-100. |
| [6] | ZHAO G Y, ZHANG C H, QIN S D, et al.Effect of interaction between potassium and structural promoters on Fischer-Tropsch performance in iron-based catalysts. J. Mol. Catal. A: Chem., 2008, 286: 137-142. |
| [7] | BORG Ø, HAMMER N, ERI S, et al. Fischer-Tropsch synthesis over un-promoted and Re-promotedg-Al2O3 supported cobalt catalysts with different pore sizes. Catal. Today, 2009, 142: 70-77. |
| [8] | DORNER R W, HARDY D R, WILLIAMS F W, et al.K and Mn doped iron-based CO2 hydrogenation catalysts: detection of KAlH4 as part of the catalyst's active phase. Appl. Catal. A: General, 2010, 373: 112-121. |
| [9] | HE XUE-ZHI, LI BING-JIE, WU ZHI-JIAN, et al. The preparation of layered double metals hydroxides Zn(Cu)/Al-LDHs and the photocatalytic reduction of CO2. J. Mol. Catal (China), 2013, 27(1): 70-75. |
| [10] | KONG X Q, TANG X J, XU S, et al.Preparation CuO-ZnO/Al2O3 by Sol-Gel auto-combustion method and its catalytic property for methanol synthesis from CO2 hydrogen. J. Mol. Catal.(China), 2013, 27(2): 159-165. |
| [11] | ZHANG Y J, DENG J L, ZHANG S J, et al.Investigation on CuO-ZnO-Al2O3/HZSM-5 catalyst for synthesis of dimethyl ether from CO2 hydrogen. J. Mol. Catal.(China), 2013, 27(3): 235-241. |
| [12] | ROY S C, VARGHESE O K, PAULOSE M.Toward solar fuels: photocatalytic conversion of carbon dioxide to hydrocarbons. ACS Nano, 2010, 4(3): 1259-1278. |
| [13] | DONG H Z, YIN X H, SUI D D, et al.Calculation of CO2 adsorption on SrTiO3(100) with density funcational theory. J. Mol. Catal.(China), 2012, 26(6): 554-559. |
| [14] | XIE S J, WANG Y, ZHANG Q H.Photocatalytic reduction of CO2 with H2O: significant enhancement of the activity of Pt-TiO2 in CH4 formation by addition of MgO. Chem. Commun., 2013, 49: 2451-2453. |
| [15] | FUJISHIMA A, HONDA K.Electrochemical photolysis of water at a semiconductor electrode. Nature, 1972, 238(5358): 37-38. |
| [16] | NOZIK A J.Photoelectrolysis of water using semiconducting TiO2 crystals. Nature, 1975, 257(5525): 383-386. |
| [17] | INOUE T, FUJISHIMA A, KONISHI S.Photoelectrocatalytic reduction of carbon dioxide in aqueous suspensions of semiconductor powders. Nature, 1979, 277: 637-638. |
| [18] | KONG D.Electrodeposited Ag nanoparticles on TiO2 nanorods for enhanced UV visible light photoreduction CO2 to CH4. Appl. Surf. Sci., 2013, 277: 105-110. |
| [19] | ZHANG Q H, HAN W D, HONG Y J.Photocatalytic reduction of CO2 with H2O on Pt-loaded TiO2 catalyst. Catal. Today, 2009, 148: 335-340. |
| [20] | THAMPI K R, KIWI J, GRATZEL M.Methanation and photo-methanation of carbon dioxide at room temperature and atmospheric pressure. Nature, 1987, 327: 506-508. |
| [21] | LI X K, ZHUANG Z J, LI W. Photocatalytic reduction of CO2 over noble metal-loaded and nitrogen-doped mesoporous TiO2. Appl. Catal. A: General, 2012, 429-430: 31-38. |
| [22] | YU K P, YU W Y, KUO M C, et al.Pt/titania-nanotube: a potential catalyst for CO2 adsorption and hydrogenation. Appl. Catal. B: Environ., 2008, 84: 112-118. |
| [23] | JACQUEMIN M, BEULS A, RUIZ P.Catalytic production of methane from CO2 and H2 at low temperature: insight on the reaction mechanism. Catal. Today, 2010, 157: 462-466. |
| [24] | SCHILD C, WOKAUN A, BAIKER A.On the mechanism of CO and CO2 hydrogenation reactions on zirconia-supported catalysts: a diffuse reflectance FTIR study Part II. Surface species on copper/zirconia catalysts: implications for methanol synthesis selectivity. J. Mol. Catal., 1990, 63: 243-254. |
| [25] | LO C C, HUNG C H, YUAN C S.Photoreduction of carbon dioxide with H2 and H2O over TiO2 and ZrO2 in a circulated photocatalytic reactor. Solar Energy Materials & Solar Cells, 2007, 91: 1765-1774. |
| [26] | DIMITRIJEVIC N M, VIJAYAN B K, POLUEKTOV O G.Role of water and carbonates in photocatalytic transformation of CO2 to CH4 on titania. J. Am. Chem. Soc., 2011, 133: 3964-3971. |
| [27] | VARGHESE O K, PAULOS M, LATEMPA T J, et al.High-rate solar photocatalytic conversion of CO2 and water vapor to hydrocarbon fuels. Nano Lett., 2009, 9(2): 731-737. |
| [28] | ABE T, TANIZAWA M, WATANABE K, et al.CO2 methanation property of Ru nanoparticle-loaded TiO2 prepared by a polygonal barrel-sputtering method. Energy Environ. Sci., 2009, 2: 315-321. |
| [29] | JIANG QI, ZHU ZHI-CHEN, HUANG ZHONG-TAO. The catalytic activity of supported Ru catalyst for the methanation of CO2. Journal of South China University of Technology (Natural Science), 1996, 24(12): 109-114. |
| [30] | LI BO, LU GONG-XUAN. Cosensitized TiO2 with different dyes for water splitting to hydrogen under visible light—structural similarity of dyes and their dual promoting effect. J. Mol. Catal (China), 2013, 27(4): 181-191. |
| [31] | WU YU-QI, LU GONG-XUAN, ZHOU QUAN, et al. Hydrogen production by Pt/TiO2 photocatalytic reforming of ethanol. J. Mol. Catal (China), 2004, 16(2): 101-106. |
| [32] | ZHEN WEN-LONG, LI BO, LU GONG XUAN, et al. Enhancing catalytic activity and stability for CO2 methanation on Ni-Ru/γ-Al2O3 via modulating impregnation sequence and controlling surface active species. RSC Adv., 2014, 4: 16472-16479. |
| [33] | ELMASIDES C, KONDARIDES D I, GRULNERT W, et al.XPS and FTIR study of Ru/Al2O3 and Ru/TiO2 catalysts: reduction characteristics and interaction with a methane-oxygen mixture. J. Phys. Chem. B., 1999, 103: 5227-5239. |
| [34] | ZHAI Q G, XIE S J, FAN W Q.Photocatalytic conversion of carbon dioxide with water into methane: Platinum and Copper(I) Oxide co-catalysts with a core-Shell structure. Angew. Chem. Int. Ed., 2013, 52: 5776-5779. |
| [35] | FRESE K W, LEACH S.Electrochemical reduction of carbon dioxide to methane, methanol, and CO on Ru electrodes. J. Electrochem. Soc., 1985, 132: 259-260. |
| [36] | WISE H, MCCARTHY J G.Thermodynamic properties of surface carbon on Ruthenium. Surface Sci., 1983, 133: 311-320. |
| [37] | SOLYMOSI F, ERDOHELYI A, KOCSIS M.Methanation of CO2 on supported Ru catalysts. J. Chem. Soc., Faraday Trans., 1981, 77(1): 1003-1012. |
| [38] | PRAIRIE M R, RENKEN A, HIGHFIELD J G, et al.A fourier transform infrared spectroscopic study of CO2 methanation on supported ruthenium. J. Catal., 1991, 129(1): 130-144. |
| [39] | MICHEL MARWOOD, RALF DOEPPER, ALBERT RENKEN. In-situ surface and gas phase analysis for kinetic studies under transient conditions The catalytic hydrogenation of CO2. Appl. Catal. A: General., 1997, 151: 223-246. |
| [1] | TUERHONG Munire, ZHAO Honggang, MA Yuhua, QI Xianhui, LI Yuchen, YAN Chenxiang, LI Jiawen, CHEN Ping. Construction and Photocatalytic Activity of Monoclinic Tungsten Oxide/Red Phosphorus Step-scheme Heterojunction [J]. Journal of Inorganic Materials, 2023, 38(6): 701-707. |
| [2] | SUN Qiangqiang, CHEN Zixuan, YANG Ziyue, WANG Yimeng, CAO Baoyue. Amorphous Vanadium Oxide Loaded by Metallic Nickel-copper towards High-efficiency Electrocatalyzing Hydrogen Production [J]. Journal of Inorganic Materials, 2023, 38(6): 647-655. |
| [3] | WU Rui, ZHANG Minhui, JIN Chenyun, LIN Jian, WANG Deping. Photothermal Core-Shell TiN@Borosilicate Bioglass Nanoparticles: Degradation and Mineralization [J]. Journal of Inorganic Materials, 2023, 38(6): 708-716. |
| [4] | WU Shuang, GOU Yanzi, WANG Yongshou, SONG Quzhi, ZHANG Qingyu, WANG Yingde. Effect of Heat Treatment on Composition, Microstructure and Mechanical Property of Domestic KD-SA SiC Fibers [J]. Journal of Inorganic Materials, 2023, 38(5): 569-576. |
| [5] | MA Xiaosen, ZHANG Lichen, LIU Yanchao, WANG Quanhua, ZHENG Jiajun, LI Ruifeng. 13X@SiO2: Synthesis and Toluene Adsorption [J]. Journal of Inorganic Materials, 2023, 38(5): 537-543. |
| [6] | HE Danqi, WEI Mingxu, LIU Ruizhi, TANG Zhixin, ZHAI Pengcheng, ZHAO Wenyu. Heavy-Fermion YbAl3 Materials: One-step Synthesis and Enhanced Thermoelectric Performance [J]. Journal of Inorganic Materials, 2023, 38(5): 577-582. |
| [7] | WU Zhen, LI Huifang, ZHANG Zhonghan, ZHANG Zhen, LI Yang, LAN Jianghe, SU Liangbi, WU Anhua. Growth and Characterization of CeF3 Crystals for Magneto-optical Application [J]. Journal of Inorganic Materials, 2023, 38(3): 296-302. |
| [8] | SUN Han, LI Wenjun, JIA Zixuan, ZHANG Yan, YIN Liying, JIE Wanqi, XU Yadong. Effect of ACRT Technology on the Large Size ZnTe Crystals Grown by Solution Method and Corresponding Terahertz Properties [J]. Journal of Inorganic Materials, 2023, 38(3): 310-315. |
| [9] | ZHANG Wanwen, LUO Jianqiang, LIU Shujuan, MA Jianguo, ZHANG Xiaoping, YANG Songwang. Zirconia Spacer: Preparation by Low Temperature Spray-coating and Application in Triple-layer Perovskite Solar Cells [J]. Journal of Inorganic Materials, 2023, 38(2): 213-218. |
| [10] | JING Kaikai, GUAN Haoyang, ZHU Siyu, ZHANG Chao, LIU Yongsheng, WANG Bo, WANG Jing, LI Mei, ZHANG Chengyu. Tensile Creep Behavior of Cansas-II SiCf/SiC Composites at High Temperatures [J]. Journal of Inorganic Materials, 2023, 38(2): 177-183. |
| [11] | YU Yefan, XU Ling, NI Zhongbing, SHI Dongjian, CHEN Mingqing. Prussian Blue Modified Biochar: Preparation and Adsorption of Ammonia Nitrogen from Sewage [J]. Journal of Inorganic Materials, 2023, 38(2): 205-212. |
| [12] | WU Xuetong, ZHANG Ruofei, YAN Xiyun, FAN Kelong. Nanozyme: a New Approach for Anti-microbial Infections [J]. Journal of Inorganic Materials, 2023, 38(1): 43-54. |
| [13] | DU Qiujing, LIU Tianzhi, CHEN Jufeng, CHEN Hangrong. Construction of Prussian Blue Fluorescent Nanoprobe for Specific Detection of HClO in Cancer Cells [J]. Journal of Inorganic Materials, 2023, 38(1): 55-61. |
| [14] | ZHANG Jiaqiang, ZOU Xinlei, WANG Nengze, JIA Chunyang. Zn-Fe PBA Films by Two-step Electrodeposition Method: Preparation and Performance in Electrochromic Devices [J]. Journal of Inorganic Materials, 2022, 37(9): 961-968. |
| [15] | CHEN Yongqiang, WANG Yixue, ZHANG Fan, LI Hongxia, DONG Binbin, MIN Zhiyu, ZHANG Rui. Preparation of Special Ceramics by Microwave Heating: a Review [J]. Journal of Inorganic Materials, 2022, 37(8): 841-852. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||