
Journal of Inorganic Materials ›› 2014, Vol. 29 ›› Issue (7): 747-752.DOI: 10.3724/SP.J.1077.2014.13514
• Orginal Article • Previous Articles Next Articles
HU Li-Na, ZHAO Xiang-Yu, LI Jia-Jia, YANG-Meng, MA Li-Qun, ZHANG Li-Yan
Received:2013-10-09
Revised:2013-11-21
Published:2014-07-20
Online:2014-06-20
About author:HU Li-Na. E-mail: hlna_nina@163.com
Supported by:CLC Number:
HU Li-Na, ZHAO Xiang-Yu, LI Jia-Jia, YANG-Meng, MA Li-Qun, ZHANG Li-Yan. Hydrothermal Preparation and Electrochemical Properties of Co-S alloys[J]. Journal of Inorganic Materials, 2014, 29(7): 747-752.
Add to citation manager EndNote|Ris|BibTeX
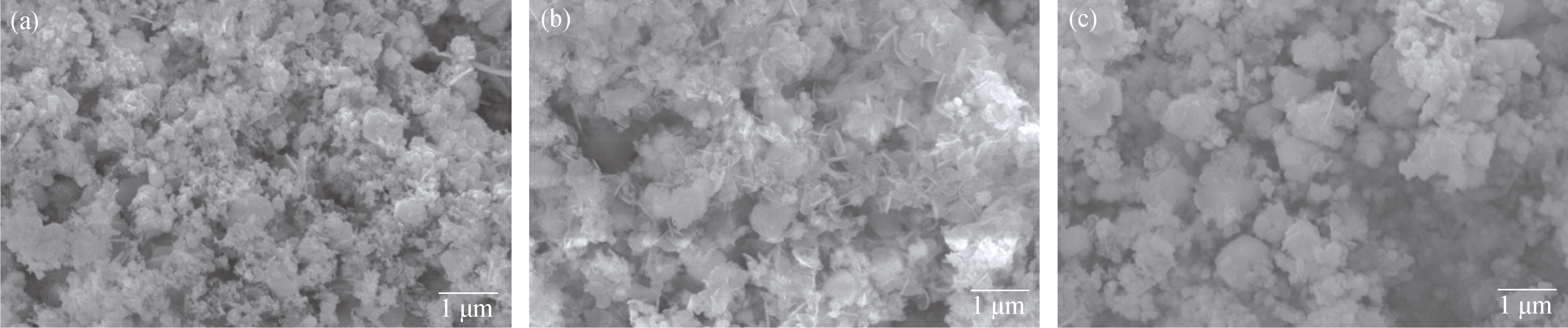
Fig. 5 SEM images of the Co-S alloys prepared with 70% packing volumes after heat-treatment at different temperatures for 12 h (a) 150℃; (b) 160℃; (c) 170℃
| [1] | TOLLEFSON J. Hdrogen vehicles: fuel of the future?Nature, 2010, 464(7293): 1262-1264. |
| [2] | ZHAO X Y, MA L Q, SHEN X D. Co-based anode materials for alkaline rechargeable Ni/Co batteries: a review. Journal of Materials Chemistry, 2012, 22(2): 277-285. |
| [3] | LIU Y, WANG Y J, XIAO L L, et al. Structure and electrochemical behaviors of a series of Co-B alloys. Electrochimica Acta, 2008, 53(5): 2265-2271. |
| [4] | WANG Y, LEE J M, WANG X. An investigation of the origin of the electrochemical hydrogen storage capacities of the ball-milled Co-Si composites. International Journal of Hydrogen Energy, 2010, 35(4): 1669-1673. |
| [5] | ZHAO D J, ZHANG S Z, YIN G P, et al. Tungsten doped Co-Se nanocomposites as an efficient non precious metal catalyst for oxygen reduction. Electrochimica Acta, 2013, 91: 179-184. |
| [6] | CAO Y L, ZHOU W C, LI X Y, et al. Electrochemical hydrogen storage behaviors of ultrafine Co-P particles prepared by direct ball-milling method. Electrochimica Acta, 2006, 51(20): 4285-4290. |
| [7] | WANG J, NG S H, WANG G X, et al. Synthesis and characterization of nanosize cobalt sulfide for rechargeable lithium batteries. Journal of Power Sources, 2006, 159(1): 287-290. |
| [8] | LU Z W, YAO S M, LI G R, et al. Microstructure and electtrochemical properties of the Co-BN composites. Electrochimica Acta, 2008, 53: 2369-2375. |
| [9] | YAO S M, XI K, LI G R, et al. Preparation and electrochemical properties of Co-Si3N4 nanocomposites. Journal of Power Sources, 2008, 184: 657-662. |
| [10] | LI J J, ZHAO X Y, MA L Q, et al. Effect of mechanical milling on structure, morphology and electrochemical performance of zinc oxide powders. Journal of Inorganic Materials, 2012, 27(6): 580-584. |
| [11] | HAMPSEY J E, CASTRO C L D, MCCAUGHEY B, et al. Preparation of micrometer-to sub-micrometer-sized nanostructured silica particles using high-energy ball milling. Journal of the American Ceramic Society, 2004, 87(7): 1280-1286. |
| [12] | WANG Q H, JIAO L F, DU H M, et al. Electrochemical hydrogen storage property of Co-S alloy prepared by ball-milling method. International Journal of Hydrogen Energy, 2010, 35(15): 8357-8362. |
| [13] | LI W J, SHI E W, ZHONG W Z, et al. Formation of oxide dendrite crystal under hydrothermal condition. Journal of Synthetic Crystals, 1999, 28(2): 54-57. |
| [14] | WANG Q H, JIAO L F, DU H M, et al. Facile synthesis and electrochemical properties of Co-S composites as negative materials for alkaline rechargeable batteries. Electrochimica Acta, 2011, 56(21): 1106-1110. |
| [15] | 施尔畏,陈之战,元如林,等. 水热结晶学. 北京: 科学出版社, 2004: 40. |
| [16] | 徐如人,庞文琴,于吉红,等. 分子筛与多孔材料化学. 北京: 科学出版社, 2004: 202-203. |
| [17] | WANG Q H, JIAO L F, DU H M, et al. Comparison of Co-S electrodes synthesized via different methods for alkaline rechargeable batteries. Electrochimica Acta, 2011, 56(14): 4992-4995. |
| [18] | LU D S, LI W S, TAN C L, et al. Investigation of Co-B-S system as anode material for secondary alkaline battery. Electrochimica Acta, 2009, 55(1): 171-177. |
| [19] | YAO Y, ZHAO X Y, MA L Q, et al. Electrochemical properties of Co(OH)2 powders as an anode in an alkaline battery. Journal of Materials Science, 2010, 45: 3752-3756. |
| [20] | ZHAO X Y, LI J J, YAO Y, et al. Electrochemical redox mechanism of Co-B-H anode material and its optimization by a novel electrolyte additive. RSC Advances, 2013, 3(5): 1327-1331. |
| [21] | ZHAO X Y, MA L Q, YAO Y, et al. Electrochemical energy storage of Co powders in alkaline electrolyte. Electrochimica Acta, 2010, 55(1): 1169-1174. |
| [1] | YAO Yishuai, GUO Ruihua, AN Shengli, ZHANG Jieyu, CHOU Kuochih, ZHANG Guofang, HUANG Yarong, PAN Gaofei. In-situ Loaded Pt-Co High Index Facets Catalysts: Preparation and Electrocatalytic Performance [J]. Journal of Inorganic Materials, 2023, 38(1): 71-78. |
| [2] | ZHANG Xian, ZHANG Ce, JIANG Wenjun, FENG Deqiang, YAO Wei. Synthesis, Electronic Structure and Visible Light Photocatalytic Performance of Quaternary BiMnVO5 [J]. Journal of Inorganic Materials, 2022, 37(1): 58-64. |
| [3] | XIAO Yumin, Li Bin, QIN Lizhao, LIN Hua, LI Qing, LIAO Bin. Efficient Preparation of CuGeO3 with Controllable Morphology Using CuCl2 as Copper Source [J]. Journal of Inorganic Materials, 2021, 36(1): 69-74. |
| [4] | WANG Juhan,WEN Xiong,LIU Chengchao,ZHANG Yuhua,ZHAO Yanxi,LI Jinlin. Preparation and Fischer-Tropsch Synthesis Performance of Hierarchical Co/Al-SiO2 Catalyst [J]. Journal of Inorganic Materials, 2020, 35(9): 999-1004. |
| [5] | XU Yun-Qing,WANG Hai-Zeng. Sodium Magnesium Fluoride Particles of Different Morphologies: Prepared by EDTA-assisted Hydrothermal Method [J]. Journal of Inorganic Materials, 2019, 34(9): 933-937. |
| [6] | GOU Sheng-Lian, NAI Xue-Ying, XIAO Jian-Fei, YE Jun-Wei, DONG Ya-Ping, LI Wu. Preparation and Thermal Decomposition of Basic Magnesium Chloride Whiskers [J]. Journal of Inorganic Materials, 2019, 34(7): 781-785. |
| [7] | Wei LIU, Kai ZHENG, Dong-Hong WANG, Yi-San LEI, Huai-Lin FAN. Co3O4 Nanowire Arrays@Activated Carbon Fiber Composite Materials: Facile Hydrothermal Synthesis and Its Electrochemical Application [J]. Journal of Inorganic Materials, 2019, 34(5): 487-492. |
| [8] | Xiao-Jing FENG, Gong-Kai WANG, Xiao-Ran WANG, Jun HE, Xin WANG, Hui-Fen PENG. Electrochemical Property of Cr 3+ Doped LiSn2(PO4)3 Anode Material [J]. Journal of Inorganic Materials, 2019, 34(4): 358-364. |
| [9] | WANG Wei, LUO Shi-Jie, XIAN Cong, XIAO Qun, YANG Yang, OU Yun, LIU Yun-Ya, XIE Shu-Hong. Enhanced Thermoelectric Properties of Hydrothermal Synthesized BiCl3/Bi2S3 Composites [J]. Journal of Inorganic Materials, 2019, 34(3): 328-334. |
| [10] | JIANG Hai-Yan, XIA Yun-Sheng, LI Yu-Zhen. Preparation and Visible-light-driven Photocatalytic Performance of Porous Rod-like FeVO4 [J]. Journal of Inorganic Materials, 2018, 33(9): 949-955. |
| [11] | WANG Hao, LUO Yong-Chun, DENG An-Qiang, ZHAO Lei, JIANG Wan-Ting. Annealing Temperature on Structural and Electrochemical Property of Mg-free La-Y-Ni Based A2B7-type Hydrogen Storage Alloys [J]. Journal of Inorganic Materials, 2018, 33(4): 434-440. |
| [12] | ZENG Yan-Fei, XIN Guo-Xiang, BULIN Chao-Ke, ZHANG Bang-Wen. One-step Preparation and Electrochemical Performance of 3D Reduced Graphene Oxide/NiO as Supercapacitor Electrodes Materials [J]. Journal of Inorganic Materials, 2018, 33(10): 1070-1076. |
| [13] | LI Guo-Chang, WANG Ping, LIU Chang-Bo. Hydrothermal Synthesis of Whitlockite [J]. Journal of Inorganic Materials, 2017, 32(11): 1128-1132. |
| [14] | MA Fang, CUI Ming-Fang, ZHU Jian-Hua, LI Ya-Li. Porous Hydroxyapatite Microspheres Prepared by Using Poly (Allylamine Hydrochloride) and Its Application in Drug Delivery [J]. Journal of Inorganic Materials, 2017, 32(11): 1215-1222. |
| [15] | RAN Hui-Li, HUANG Hao, MA Meng-Jun, ZHAI Jin-Sheng, FAN Jia-Jie. Dye-sensitized Solar Cells Based on Double-layer Composite Film with Enhanced Photovoltaic Performance [J]. Journal of Inorganic Materials, 2017, 32(10): 1049-1054. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||