
Journal of Inorganic Materials ›› 2014, Vol. 29 ›› Issue (7): 717-721.DOI: 10.3724/SP.J.1077.2014.13564
• Orginal Article • Previous Articles Next Articles
YANG Bi-Yun1, LI Jun-Ping2, WANG Ting-Yu1, LI Rui-Xing1, FENG Zhi-Hai2, CAI Hong-Nian3
Received:2013-10-30
Revised:2013-12-03
Published:2014-07-20
Online:2014-06-20
About author:YANG Bi-Yun. E-mail: yangbiyun08@163.com
Supported by:CLC Number:
YANG Bi-Yun, LI Jun-Ping, WANG Ting-Yu, LI Rui-Xing, FENG Zhi-Hai, CAI Hong-Nian. Effects of Gel Aging on Synthesis of ZrB2 Powders[J]. Journal of Inorganic Materials, 2014, 29(7): 717-721.
Add to citation manager EndNote|Ris|BibTeX
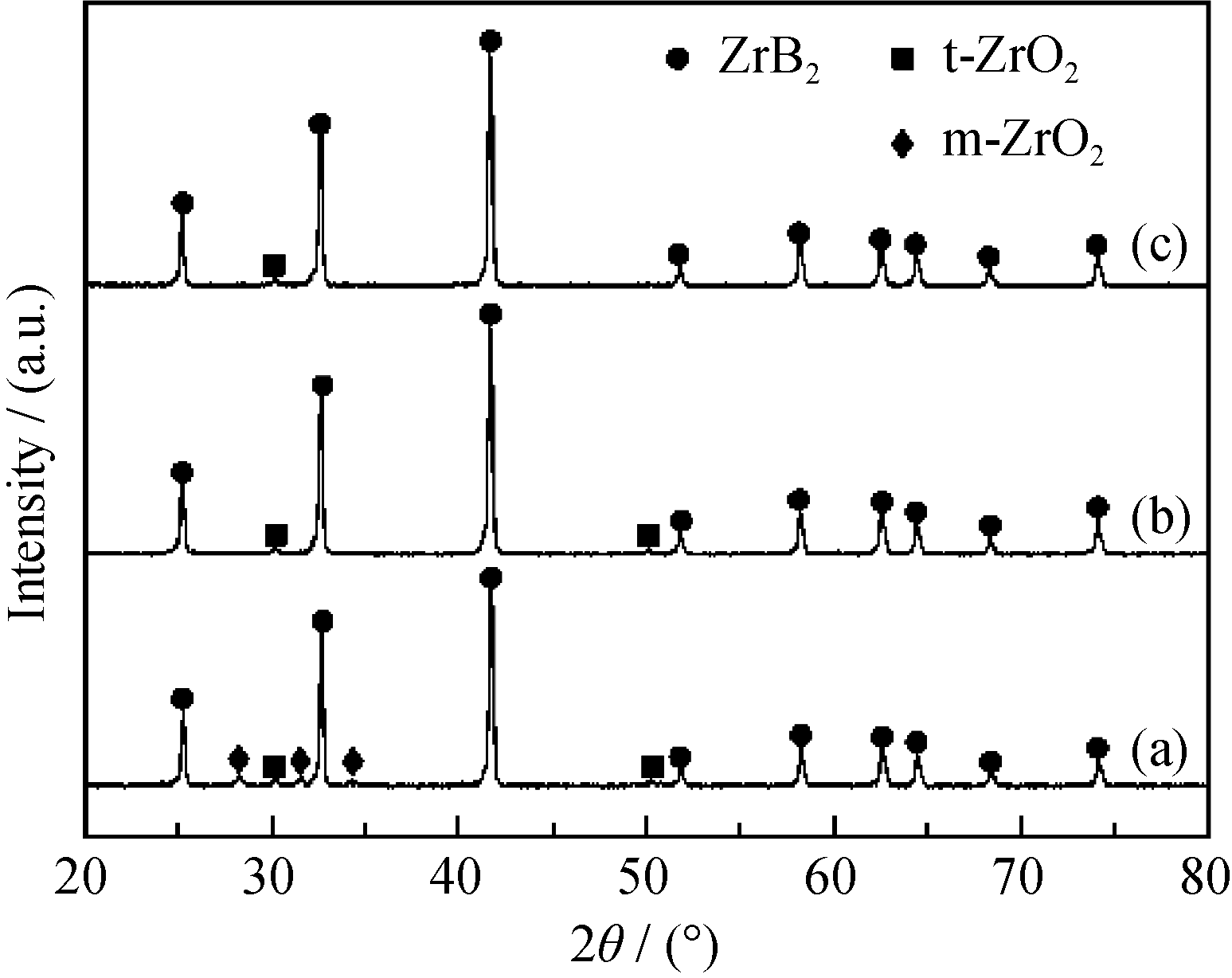
Fig. 2 XRD patterns of ZrB2 powders calcined at 1550 ℃ for 2 h using nascent state gels Molar ratios of (a) B/Zr = 3.5, C/Zr = 5; (b) B/Zr = 3.5, C/Zr = 7; (c) B/Zr = 4, C/Zr = 7
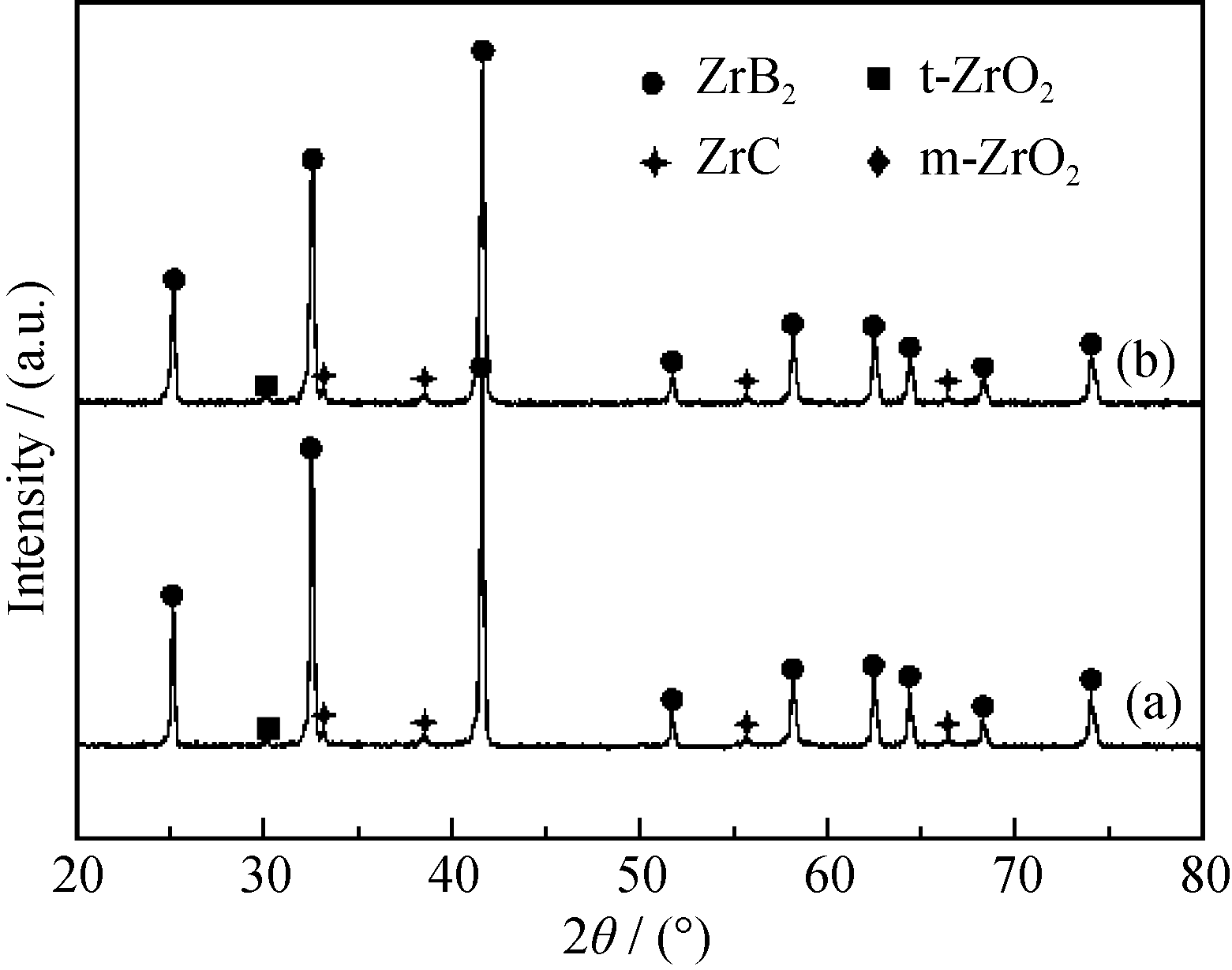
Fig. 3 XRD patterns of ZrB2 powders calcined at 1550 ℃ for 2 h using nascent state gels with n(C)/n(Zr)= 8 and different B/Zr molar ratios of (a) 3.5 and (b) 4
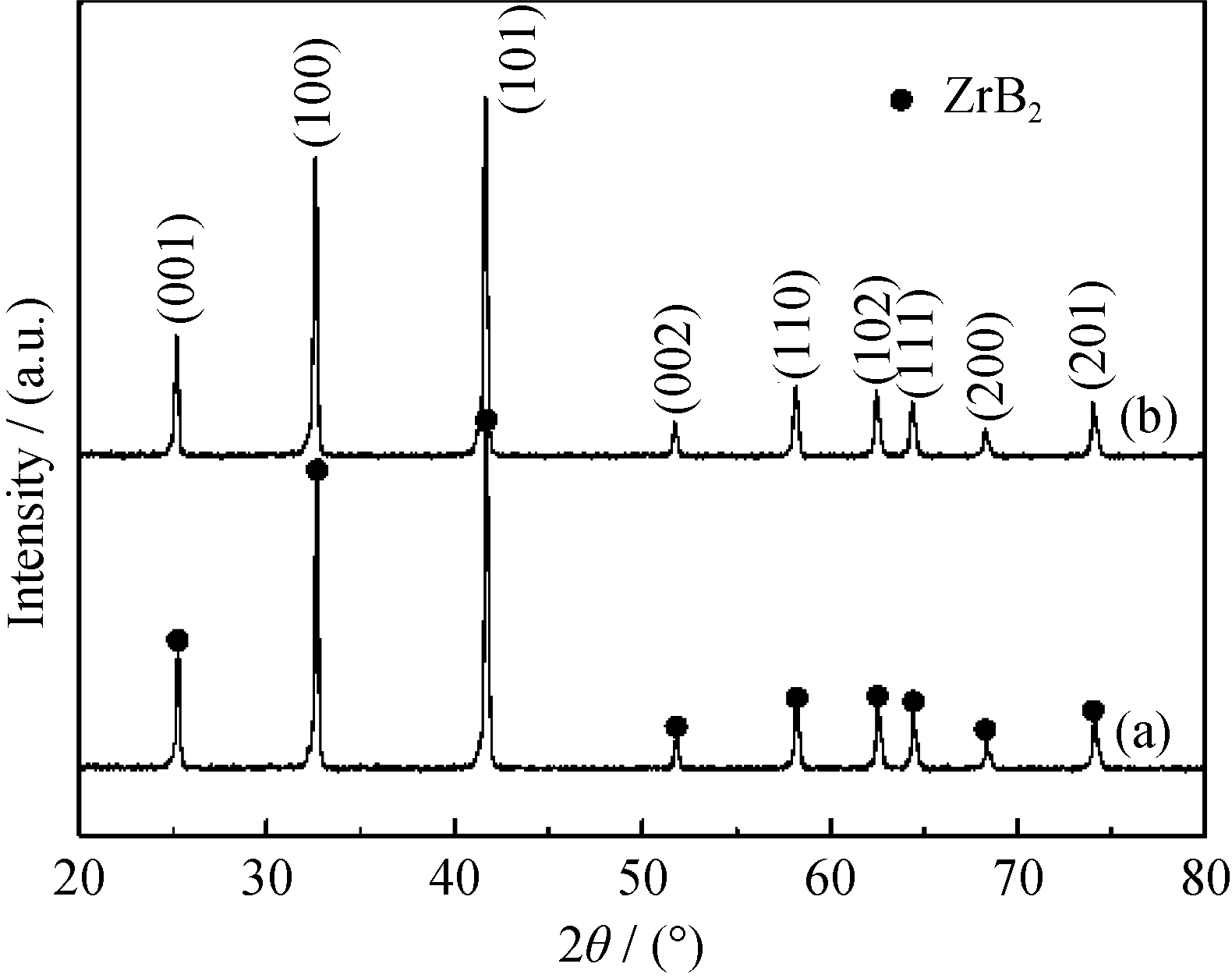
Fig. 4 XRD patterns of ZrB2 powders calcined at 1550 ℃ for 2 h using aged gels with n(C)/n(Zr) = 7 and different B/Zr molar ratios of 3.5 (a) and 4 (b)
| Sample | Gel | BET/(m2·g-1) |
|---|---|---|
| 1 | Nascent state | 15.29 |
| 2 | Aged | 2.53 |
Table1 Specific surface areas (BET) of ZrB2 powders calcined at 1550 ℃for 2 h using nascent state gels and aged gels with molar ratios of B/Zr = 3.5 and C/Zr = 7
| Sample | Gel | BET/(m2·g-1) |
|---|---|---|
| 1 | Nascent state | 15.29 |
| 2 | Aged | 2.53 |
| [1] | MORZ C. Annual mineral review: zirconium diboride. American Ceramic Society Bulletin, 1995, 73(6): 141-142. |
| [2] | FAHRENHOLTZ W G, HILMAS G E. Refractory diborides of zirconium and hafnium. Journal of the American Ceramic Society, 2007, 90(5): 1347-1364. |
| [3] | UPADHYA K, YANG J M, HOFFMANN W P. Materials for ultrahigh temperature structural applications. American Ceramic Society Bulletin, 1997, 76(12): 51-56. |
| [4] | QIU HUI-YU, GUO WEI-MING, ZOU JI, et al. ZrB2 powders prepared by boro/carbothermal reduction of ZrO2: the effects of carbon source and reaction atmosphere. Powder Technology, 2012, 217(3): 462-466. |
| [5] | CHEN LU-YANG, GU YUN-LE, YANG ZE-HENG, et al. Preparation and some properties of nanocrystalline ZrB2 powders. Scripta Materialia, 2004, 50(7): 959-961. |
| [6] | GUO SHU-QI, HU CHUN-FENG, KAGAWA Y. Mechanochemical processing of nanocrystalline zirconium diboride powder. Journal of the American Ceramic Society, 2011, 94(11): 3643-3647. |
| [7] | ÇAMURLU H E, MAGLIA F. Preparation of nano-size ZrB2 powder by self-propagating high-temperature synthesis. Journal of the European Ceramic Society, 2009, 29(8): 1501-1506. |
| [8] | BAČA Ľ, STELZER N. Adapting of Sol-Gel process for preparation of TiB2 powder from low-cost precursors. Journal of the European Ceramic Society, 2008, 28(5): 907-911. |
| [9] | YAN YONG-JIE, HUANG ZHENG-REN, LIU XUE-JIAN. Carbothermal synthesis of ultra-fine zirconium carbide powders using inorganic precursors via Sol-Gel method. Journal of Sol-Gel Science and Technology, 2007, 44(1): 81-85. |
| [10] | LI JIN-WANG, TIAN JIE-MO, DONG LI-MIN. Synthesis of SiC precursors by a two-step Sol-Gel process and their conversion to SiC powders. Journal of the European Ceramic Society, 2000, 20(11): 1853-1857. |
| [11] | XIE YAN-LI, SANDERS JR. T H, SPEYER R F. Solution-based synthesis of submicrometer ZrB2 and ZrB2-TaB2. Journal of the American Ceramic Society, 2008, 91(5): 1469-1474. |
| [12] | YAN YONG-JIE, HUANG ZHENG-REN, DONG SHAO-MING, et al. New route to synthesize ultra-fine zirconium diboride powders using inorganic-organic hybrid precursors. Journal of the American Ceramic Society, 2006, 89(11): 3585-3588. |
| [13] | LI YUN-TAO, TAO XUE-YU, QIU WEN-FENG, et al. Preparation of powdered zirconium diboride by a solution precursor convention method. Journal of Beijing University of Chemical Technology (Natural Science), 2010, 37(4): 78-82. |
| [14] | LI RUI-XING, ZHANG YUN, LOU HAI-JIE, et al. Synthesis of ZrB2 nanoparticles by Sol-Gel method. Journal of Sol-Gel Science and Technology, 2011, 58(2): 580-585. |
| [15] | LI RUI-XING, LOU HAI-JIE, YIN SHU, et al. Nanocarbon- dependent synthesis of ZrB2 in a binary ZrO2 and boron system. Journal of Alloys and Compounds, 2011, 509(34): 8581-8583. |
| [16] | ZHANG YUN, LI RUI-XING, JIANG YAN-SHAN, et al. Morphology evolution of ZrB2 nanoparticles synthesized by Sol-Gel method. Journal of Solid State Chemistry, 2011, 184(8): 2047-2052. |
| [17] | YI GUANG-HUA, SAYER M. An acetic acid/water based Sol-Gel PZT process I: modification of Zr and Ti alkoxides with acetic acid. Journal of Sol-Gel Science and Technology, 1996, 6(1): 65-74. |
| [18] | DOLLÉ M, GOSSET D, BOGICEVIC C, et al. Synthesis of nanosized zirconium carbide by a Sol-Gel route. Journal of the European Ceramic Society, 2007, 27(4): 2061-2067. |
| [1] | HE Danqi, WEI Mingxu, LIU Ruizhi, TANG Zhixin, ZHAI Pengcheng, ZHAO Wenyu. Heavy-Fermion YbAl3 Materials: One-step Synthesis and Enhanced Thermoelectric Performance [J]. Journal of Inorganic Materials, 2023, 38(5): 577-582. |
| [2] | LIU Wenlong, ZHAO Jin, LIU Juan, MAO Xiaojian, ZHANG Jian, WANG Shiwei. Microwave Drying of Spontaneous-Coagulation-Cast Wet Alumina Green Body [J]. Journal of Inorganic Materials, 2023, 38(4): 461-468. |
| [3] | JIN Xihai, DONG Manjiang, KAN Yanmei, LIANG Bo, DONG Shaoming. Fabrication of Transparent AlON by Gel Casting and Pressureless Sintering [J]. Journal of Inorganic Materials, 2023, 38(2): 193-198. |
| [4] | ZHANG Ye, ZENG Yuping. Progress of Porous Silicon Nitride Ceramics Prepared via Self-propagating High Temperature Synthesis [J]. Journal of Inorganic Materials, 2022, 37(8): 853-864. |
| [5] | ZHANG Ye, YAO Dongxu, ZUO Kaihui, XIA Yongfeng, YIN Jinwei, ZENG Yuping. Combustion Synthesis of Si3N4-BN-SiC Composites by in-situ Introduction of BN and SiC [J]. Journal of Inorganic Materials, 2022, 37(5): 574-578. |
| [6] | LIU Jinling, LIU Dianguang, REN Ke, WANG Yiguang. Research Progress on the Flash Sintering Mechanism of Oxide Ceramics and Its Application [J]. Journal of Inorganic Materials, 2022, 37(5): 473-480. |
| [7] | MENG Qing, LI Jiangtao. Hydrophobic BN Powders by Combustion Synthesis and Its Super-hydrophobic Coatings: Preparation and Property [J]. Journal of Inorganic Materials, 2022, 37(10): 1037-1042. |
| [8] | YANG Dongwang, LUO Tingting, SU Xianli, WU Jinsong, TANG Xinfeng. Unveiling the Intrinsic Low Thermal Conductivity of BiAgSeS through Entropy Engineering in SHS Kinetic Process [J]. Journal of Inorganic Materials, 2021, 36(9): 991-998. |
| [9] | LI Jiang, DING Jiyang, HUANG Xinyou. Rare Earth Doped Gd2O2S Scintillation Ceramics [J]. Journal of Inorganic Materials, 2021, 36(8): 789-806. |
| [10] | LI Ziyi, ZHANG Jiajia, ZOU Xiaoqin, ZUO Jiayu, LI Jun, LIU Yingshu, PUI David Youhong. Synthesis and Gas Separation of Chabazite Zeolite Membranes [J]. Journal of Inorganic Materials, 2021, 36(6): 579-591. |
| [11] | LIU Yong, BAI Haijun, ZHAO Qizhi, YANG Jinge, LI Yujie, ZHENG Chunman, XIE Kai. Storage Aging Mechanism of LiNi0.8Co0.15Al0.05O2/Graphite Li-ion Batteries at High State of Charge [J]. Journal of Inorganic Materials, 2021, 36(2): 175-180. |
| [12] | WANG Tingting, SHI Shumei, LIU Chenyuan, ZHU Wancheng, ZHANG Heng. Synthesis of Hierarchical Porous Nickel Phyllosilicate Microspheres as Efficient Adsorbents for Removal of Basic Fuchsin [J]. Journal of Inorganic Materials, 2021, 36(12): 1330-1336. |
| [13] | MAN Xin, WU Nan, ZHANG Mu, HE Hongliang, SUN Xudong, LI Xiaodong. Lu2O3-MgO Nano-powder: Synthesis and Fabrication of Composite Infrared Transparent Ceramics [J]. Journal of Inorganic Materials, 2021, 36(12): 1263-1269. |
| [14] | LIU Qian, WANG Jiacheng, ZHOU Zhenzhen, XU Xiaoke. Research Progress on High Throughput Parallel Synthesis of Micro-nano Powders Libraries [J]. Journal of Inorganic Materials, 2021, 36(12): 1237-1246. |
| [15] | YANG Conggang, MI Le, FENG Aihu, YU Yang, SUN Dazhi, YU Yun. Synthesis and Performance of KH-560 Modified SiO2 Insulation Coating [J]. Journal of Inorganic Materials, 2021, 36(12): 1343-1348. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||