
Journal of Inorganic Materials ›› 2014, Vol. 29 ›› Issue (7): 711-716.DOI: 10.3724/SP.J.1077.2014.13516
• Orginal Article • Previous Articles Next Articles
DOU Zhi-He, ZHANG Ting-An, WEN Ming, SHI Guan-Yong, HE Ji-Cheng
Received:2013-10-09
Revised:2014-01-23
Published:2014-07-20
Online:2014-06-20
About author:DOU Zhi-He. E-mail:douzh@smm.neu.edu.cn
CLC Number:
DOU Zhi-He, ZHANG Ting-An, WEN Ming, SHI Guan-Yong, HE Ji-Cheng. Preparation of Ultra-fine NdB6 Powders by Combustion Synthesis and Its Reaction Mechanism[J]. Journal of Inorganic Materials, 2014, 29(7): 711-716.
Add to citation manager EndNote|Ris|BibTeX
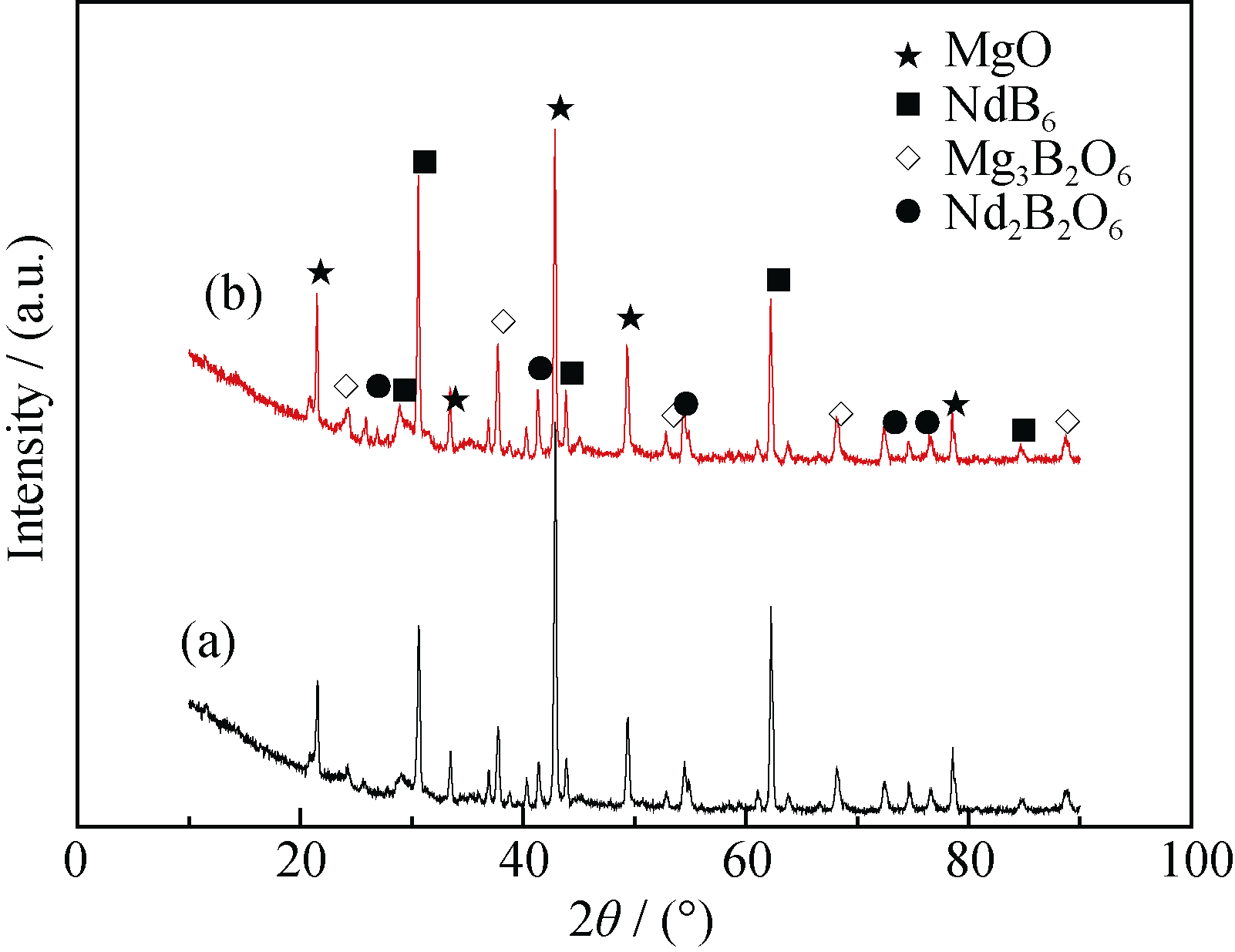
Fig. 1 XRD patterns of combustion products (a) excessive 5% of Mg in contrast to the theoretical mass ratio of reaction (1) and preparing sample pressure at 20 MPa in air; (b) excessive 10% of Mg in contrast to the theoretical mass ratio of reaction (1) and preparing sample pressure at 20 MPa in vacuum

Fig. 3 SEM images of burning products and EDS results (a) theoretical mass ratio of reaction (1); (b)~(d) excessive 5%, 10% and 20% amount of Mg, respectively, more than theoretical mass ratio of reaction (1); (e) and (f) excessive 10% amount of Mg more than theoretical mass ratio of reaction (1) and reaction pressure at 10 MPa and 20 MPa, respectively; (g) and (h) EDS results of region1 and region 2 in sample (c), respectively

Fig. 4 SEM images of NdB6 powders and EDS result The preparing pressures for samples (a)-(c) are 5, 10 and 20 MPa in atmosphere, respectively, and samples (d)-(f) are 5, 10 and 20 MPa in vacuum, respectively; (g) EDS result of region 1 in (f)
| [1] | JI X H, ZHANG Q Y, XU J Q.et al. Rare-earth hexaborides nanostructures: recent advances in materials, characterization and investigations of physical properties. Progress in Solid State Chemistry, 2011, 39(2): 51-69. |
| [2] | GYGAX F N, SCHENCK A. Dynamics of positive muons in CaB6. Journal of Alloys and Compounds, 2005, 404-406(11): 360-364. |
| [3] | BLOMBERG M K, MERISALO M J, Korsukova M, et al. Single crystal X-ray diffraction study of NdB6, YbB6 and EuB6. Journal of Alloys and Compounds, 1995, 217: 123-127. |
| [4] | KUBO Y, ASANOB S, HARIMAC H. Fermi surfaces in antiferromagnetic compounds NdIn3 and NdB6.Condensed Matter, 1993, 188(2): 132-135. |
| [5] | KANAKALA R, ESCUDERO R, ROJAS G, et al. Mechanisms of combustion synthesis and magnetic response of high-surface-area hexaboride compounds. ACS Applied Materials & Interfaces, 2011, 3(4): 1093-1100. |
| [6] | MARIAN R, JOSEF S, EVA S, et al. Heat capacity of NdB6. Journal of Magnetism and Magnetic Materials, 2007, 310: 595-597. |
| [7] | DING Q W, ZHAO Y M, XU J Q, et al. Large-scale synthesis of neodymium hexaboride nanowires by self-catalyst. Solid State Communications, 2007, 141: 53-56. |
| [8] | LU J Q, QIN J N, LU W J, et al. In situ preparation of (TiB+ TiC+Nd2O3)/Ti composites by powder metallurgy. Journal of Alloys and Compounds, 2009, 469(1/2): 116-122. |
| [9] | LIU YANG. Synthesis of NdB6 and Fabrication Techniqueof in situ (TiB+TiC+Nd2O3)/Ti Composites by Powder Metallurgy, Shanghai Jiaotong University Press, 2007: 12-25. |
| [10] | LIU Y, LU W J, QIN J N, et al. A new route for the synthesis of NdB6 powder from Nd2O3-B4C system. Journal of Alloys and Compounds, 2007, 431(1/2): 337-341. |
| [11] | ZHAO XD, LIU X Y, LIN F, et al. A new route for the synthesis of boron-rich rare-earth boride NdB6 under high pressure and high temperature. Journal of Alloys and Compounds, 1997, 249: 247-250. |
| [12] | DOU ZHIHE, ZHANG TING’AN, LIU YAN, et al. Preparation of CeB6 nano-powders by self-propagating high-temperature synthesis (SHS). Journal of Rare Earths, 2011, 29(11): 986-989. |
| [13] | ZHANG TING-AN, DOU ZHIHE. Growth mechanism of TiB2 powder prepared by SHS-metallurgy, Journal of Inorganic Material, 2006, 21(3): 583-590. |
| [14] | Li Yuzeng. Thermal Analysis. Beijing: Qinghua University Press, 1987: 207-219. |
| [1] | ZHANG Ye, YAO Dongxu, ZUO Kaihui, XIA Yongfeng, YIN Jinwei, ZENG Yuping. Combustion Synthesis of Si3N4-BN-SiC Composites by in-situ Introduction of BN and SiC [J]. Journal of Inorganic Materials, 2022, 37(5): 574-578. |
| [2] | MENG Qing, LI Jiangtao. Hydrophobic BN Powders by Combustion Synthesis and Its Super-hydrophobic Coatings: Preparation and Property [J]. Journal of Inorganic Materials, 2022, 37(10): 1037-1042. |
| [3] | GAO Wa, XIONG Yujie, WU Congping, ZHOU Yong, ZOU Zhigang. Recent Progress on Photocatalytic CO2 Reduction with Ultrathin Nanostructures [J]. Journal of Inorganic Materials, 2022, 37(1): 3-14. |
| [4] | ZHENG Yanning, JI Junrong, LIANG Xueling, LAI Zhengjie, CHENG Qifan, LIAO Dankui. Performance of Nitrogen-doped Hollow Carbon Spheres as Oxidase Mimic [J]. Journal of Inorganic Materials, 2021, 36(5): 527-534. |
| [5] | XU Yun-Qing,WANG Hai-Zeng. Sodium Magnesium Fluoride Particles of Different Morphologies: Prepared by EDTA-assisted Hydrothermal Method [J]. Journal of Inorganic Materials, 2019, 34(9): 933-937. |
| [6] | YU Yin-Hu, WANG Tao, LIAO Qiu-Ping, MIAO Run-Jie, PAN Jian-Feng, ZHANG Du-Bao. Low-temperature Solid-state Synthesis of Nanometer TiB2-TiC Composite Powder [J]. Journal of Inorganic Materials, 2016, 31(3): 324-328. |
| [7] | YU Yin-Hu, WANG Tao, ZHANG Hong-Min, ZHANG Du-Bao, PAN Jian-Feng. Low Temperature Combustion Synthesis of TiC Powder Induced by PTFE [J]. Journal of Inorganic Materials, 2015, 30(3): 272-276. |
| [8] | GUO Xiang-Xin, HUANG Shi-Ting, ZHAO Ning, CUI Zhong-Hui, FAN Wu-Gang, LI Chi-Lin, LI Hong. Rapid Development and Critical Issues of Secondary Lithium-air Batteries [J]. Journal of Inorganic Materials, 2014, 29(2): 113-123. |
| [9] | LA Pei-Qing, HAN Shao-Bo, LU Xue-Feng, WEI Yu-Peng. Effects of the Diluent Content on Microstructure of Submicron ZrB2 by Combustion Synthesis [J]. Journal of Inorganic Materials, 2014, 29(2): 191-196. |
| [10] | YANG Zeng-Chao, LIU Guang-Hua, XU Li-Hua, LI Jiang-Tao, GUO Shi-Bin. Investigation on Crystallization Behavior of Y2O3-Al2O3-SiO2 Glass Prepared by High-gravity Combustion Synthesis [J]. Journal of Inorganic Materials, 2013, 28(7): 780-784. |
| [11] | SHI Xiao-Rui, WANG Qun, LV Ling-Yuan, LI Yang, YU Xiao, CHEN Gang. Controllable Synthesis and Electrical Conductivities of Cu7Te4 Nanostructures [J]. Journal of Inorganic Materials, 2012, 27(4): 433-438. |
| [12] | CHEN Yi-Xiang, YANG Jian-Hui, JIANG Zhi-Jun, LIN Zhi-Ming, LI Jiang-Tao. Combustion Synthesis of α-Si3N4 Powder Using AC as Additive [J]. Journal of Inorganic Materials, 2012, 27(2): 169-173. |
| [13] | MU Yun-Chao, LIANG Bao-Yan, GUO Ji-Feng. Reaction Mechanism of Ti3SiC2 Formed on the Diamond [J]. Journal of Inorganic Materials, 2012, 27(10): 1099-1104. |
| [14] | ZOU Xiao-Qing, ZHOU Guo-Hong, YI Hai-Lan, YANG Yan, WANG Shi-Wei. Fabrication of Transparent Y2Hf2O7 Ceramic from Combustion Synthesized Powders [J]. Journal of Inorganic Materials, 2011, 26(9): 929-932. |
| [15] | XU Xiu-Hua,XIAO Han-Ning,GUO Wen-Ming,GAO Peng-Zhao,PENG Su-Hua. Preparation and Reaction Mechanism of LaB6 Powder by Solid-state Reaction at Atmospheric Pressure [J]. Journal of Inorganic Materials, 2011, 26(4): 417-421. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||