
Journal of Inorganic Materials ›› 2014, Vol. 29 ›› Issue (6): 661-666.DOI: 10.3724/SP.J.1077.2014.14031
• Orginal Article • Previous Articles Next Articles
LI Yun-Jiao1,2, XU Hu1,2, KONG Long1,2, LI Hua-Cheng2, LI Chun-Xia2, ZHANG Xian-Zhen1, HAN Qiang1,2
Received:2014-01-13
Published:2014-06-20
Online:2014-05-27
Supported by:CLC Number:
LI Yun-Jiao, XU Hu, KONG Long, LI Hua-Cheng, LI Chun-Xia, ZHANG Xian-Zhen, HAN Qiang. Synthesis and Electrochemical Characterizations of Co-doped Lithium
Manganese Oxide Spinel Li1.035Mn1.965O4[J]. Journal of Inorganic Materials, 2014, 29(6): 661-666.
Add to citation manager EndNote|Ris|BibTeX
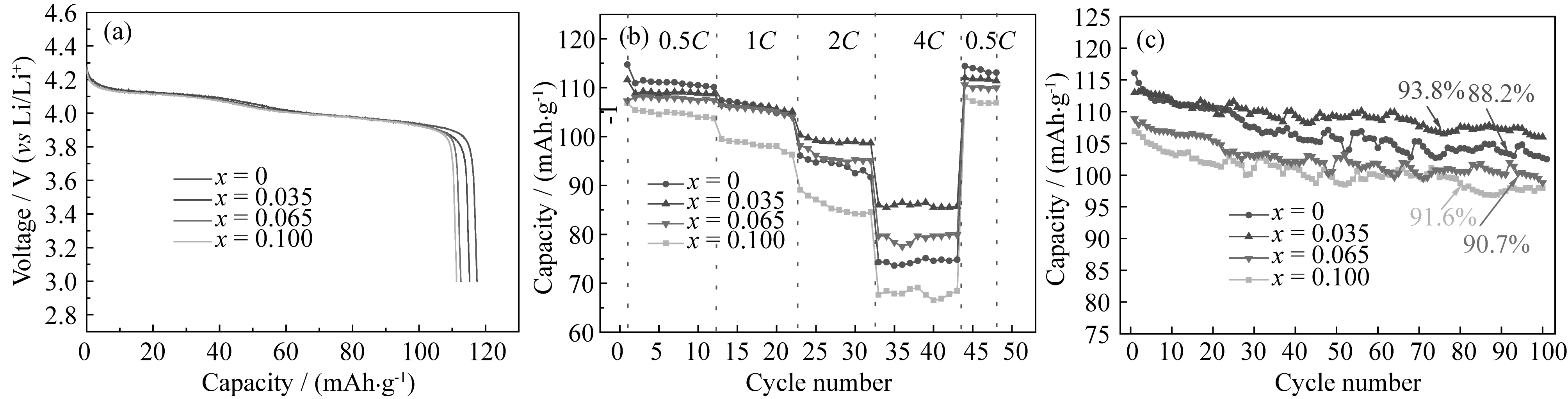
Fig. 5 Discharge curves at 0.1C rate (a), discharge capacity with cycling number at different current rates (b) and cycling <br/>performance at 0.5C (c) of Li1.035CoxMn1.965-x
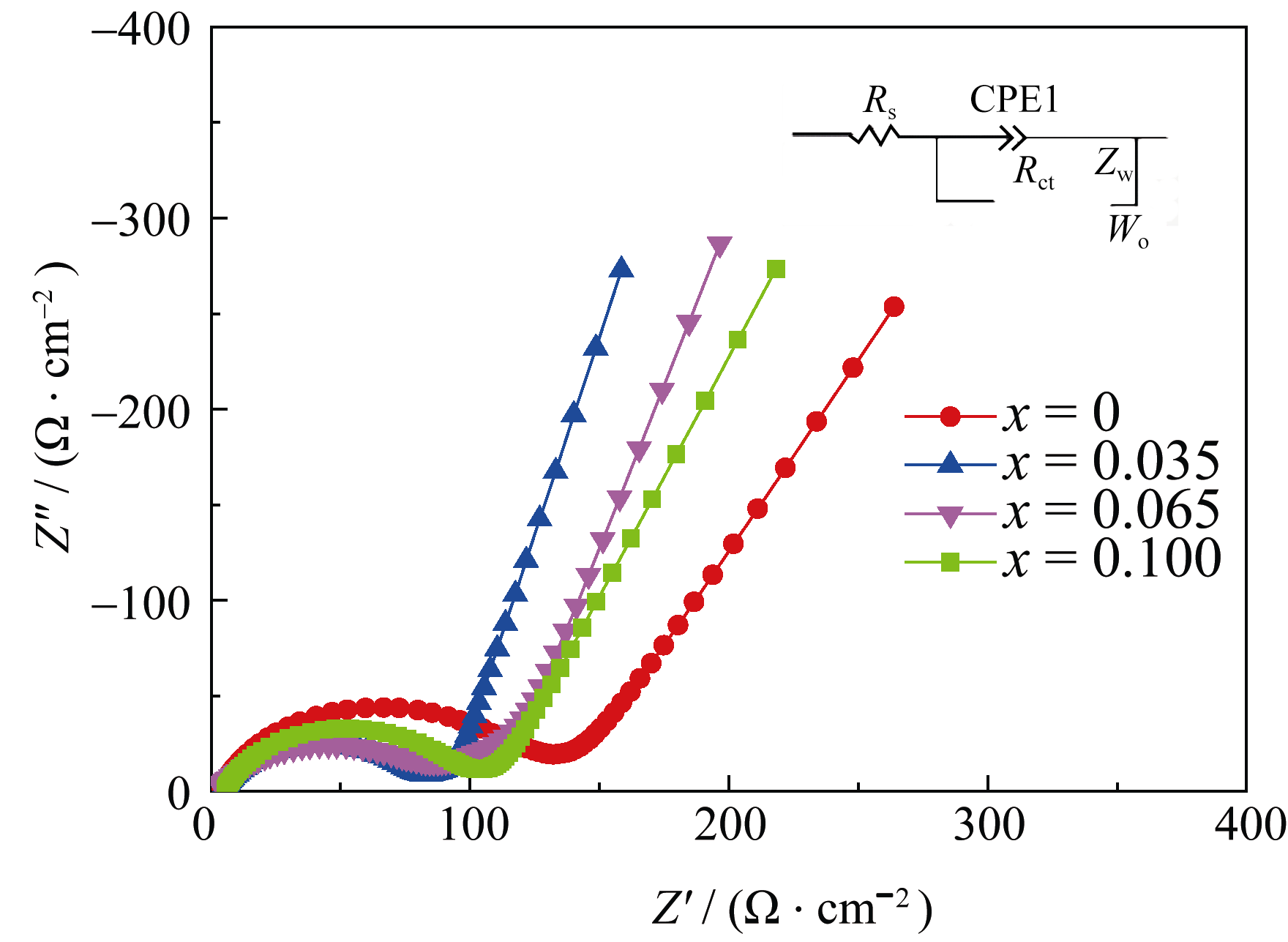
Fig. 6 Electrochemical impedance spectra (EIS) of the samples in the discharged state (about 3.0 V, vs Li+/Li) after 3 cycles with an equivalent circuit diagram inserted
| Samples | Rs/Ω | Rct/Ω | Wo-T | L/nm | DLi+/(cm2•s-1) |
|---|---|---|---|---|---|
| x = 0 | 4.32 | 129.40 | 1.976 | 596 | 1.81×10-9 |
| x = 0.035 | 3.28 | 79.12 | 0.121 | 621 | 3.19×10-8 |
| x = 0.065 | 3.39 | 91.20 | 0.286 | 618 | 1.37×10-8 |
| x = 0.100 | 3.85 | 102.00 | 0.547 | 609 | 6.85×10-9 |
Table 1 The impedance parameters of Li1.035CoxMn1.965-xO4 samples
| Samples | Rs/Ω | Rct/Ω | Wo-T | L/nm | DLi+/(cm2•s-1) |
|---|---|---|---|---|---|
| x = 0 | 4.32 | 129.40 | 1.976 | 596 | 1.81×10-9 |
| x = 0.035 | 3.28 | 79.12 | 0.121 | 621 | 3.19×10-8 |
| x = 0.065 | 3.39 | 91.20 | 0.286 | 618 | 1.37×10-8 |
| x = 0.100 | 3.85 | 102.00 | 0.547 | 609 | 6.85×10-9 |
| [1] | LI J, KLOPSCH M C, STAN S. Synthesis and electrochemical performance of the high voltage cathode material Li[Li0.2Mn0.56 Ni0.16Co0.08]O2 with improved rate capability .Journal of Power Sources, 2011, 196(10): 4821-4825. |
| [2] | PARK O K, CHO Y, LEE S, et al. Who will drive electric vehicles, olivine or spinel?Energy & Environmental Science, 2011, 4: 1621-1633. |
| [3] | TARASCON J M, ARMAND M. Issues and challenges facing rechargeable lithium batteries. Nature, 2001, 414: 359-367. |
| [4] | KANG K, MENG Y S, BRÉGER J, et al. Electrodes with high power and high capacity for rechargeable lithium batteries. Science, 2006, 311: 977-980. |
| [5] | SIMMEN F, HINTENNACH A, HORISBERGER M, et al. Aspects of the surface layer formation on Li1+xMn2O4-δ during electrochemical cycling. Journal of The Electrochemical Society, 2010, 157(9): A1026-A1029. |
| [6] | XIE J, TANAKA T, IMANISHI N. Li-ion transport kinetics in LiMn2O4 thin films prepared by radio frequency magnetron sputtering. Journal of Power Sources, 2008, 180(1): 576-581. |
| [7] | WANG Y, CAO G Z. Developments in nanostructured cathode materials for high-performance lithium-ion batteries. Advanced Materials, 2008, 20(12): 2251-2269. |
| [8] | GUO Y G, HU Y S, SIGLE W. Superior electrode performance of nanostructured mesoporous TiO2 (anatase) through efficient hierarchical mixed conducting networks. Advanced Materials, 2007, 19(16): 2087-2091. |
| [9] | LIDDLE B J, COLLINS S M, BARTLETT B M. A new one- pot hydrothermal synthesis and electrochemical characterization of Li1+xMn2-yO4 spinel structured compounds.- Energy & Environmental Science, 2010, 3: 1339-1346. |
| [10] | ARILLO M A, CUELLO G, LÓPEZ M L. Structural characterisation and physical properties of LiMMnO4 (M=Cr, Ti) spinels. Solid State Sciences, 2005, 7(1): 25-32. |
| [11] | HAYASHI N, IKUTA H, WAKIHARA M. Cathode of LiMgyMn2- yO4 and yMn2-yO4-δ spinel phases for lithium secondary batteries. Journal of The Electrochemical Society, 1999, 146(4): 1351-1354. |
| [12] | MANDAL S, ROJAS R M, AMARILLA J M, et al. High temperature Co-doped LiMn2O4-based spinels. Structural, electrical, and electrochemical characterization. Chemistry of Materials, 2002, 14(4): 1598-1605. |
| [13] | IVANOVA S, ZHECHEVA E, STOYANOVA R, et al. High- voltage LiNi1/2Mn3/2O4 spinel: cationic order and particle size distribution. The Journal of Physical Chemistry C, 2011, 115(50): 25170-25182 . |
| [14] | XIONG L L, XU Y L, ZHANG C, et al. Electrochemical properties of tetravalent Ti-doped spinel LiMn2O4. Journal of Solid State Electrochemistry, 2011, 15(6): 1263-1269. |
| [15] | MYUNG S T, KOMABA S, HOSOYA K, et al. Synthesis of LiNi0.5Mn0.5-xTixO2 by an emulsion drying method and effect of Ti on structure and electrochemical properties. Chemistry of Materials, 2005, 17(9): 2427-2435. |
| [16] | TSUJI T, UMAKOSHI H, YAMAMURA Y. Thermodynamic properties of undoped and Fe-doped LiMn2O4 at high temperature. Journal of Physics and Chemistry of Solids, 2005, 66(2/3/4): 283-287. |
| [17] | LI G H, IKUTA H, UCHIDA T. The spinel phases LiMyMn2-yO4 (M=Co, Cr, Ni) as the cathode for rechargeable lithium batteries. Journal of The Electrochemical Society, 1996, 143(1): 178-182. |
| [18] | SUNG W O, PARK S H, JUNG E, et al., Improved high-rate capability of Li [Ni0.5CoyMn1.5-y]O4-zFz spinel materials for 5 V lithium secondary batteries. Journal of Industrial and Engineering Chemistry, 2007, 13: 1174-1179. |
| [19] | THIRUNAKARAN R, SIVASHANMUGAM A, GOPUKUMAR S. Electrochemical behaviour of nano-sized spinel LiMn2O4 and LiAlxMn2-xO4 (x=Al: 0.00-0.40) synthesized via fumaric acid- assisted Sol-Gel synthesis for use in lithium rechargeable batteries. Journal of Physics and Chemistry of Solids, 2008, 69(8): 2082-2090. |
| [20] | LIU Z L,WANG H B, FANG L. Improving the high-temperature performance of LiMn2O4 spinel by micro-emulsion coating of LiCoO2. Journal of Power Sources, 2002, 104(1): 101-107. |
| [21] | YOSHIMURA M, BYRAPPA K. Hydrothermal processing of materials: past, present and future. Journal of Materials Science, 2008, 43(7): 2085-2103. |
| [22] | LI Y J, KONG L, XI X M, et al. Hydrothermal Preparation and Characterization of LiMn2O4 for Li-ion Battery Application. Proceedings of COM 2012, Niagara Falls, Ontario, Canada, 2012. |
| [23] | AKIMOTO J, TAKAHASHI Y, GOTOH Y, et al. Synthesis, crystal structure, and magnetic property of delithiated LixMnO2 (x < 0.1) single crystals: A novel disordered rocksalt-type manganese dioxide. Chemical of Materials, 2003, 15(15): 2984-2990. |
| [24] | HE X M, LI J J, CAI Y. Preparation of co-doped spherical spinel LiMn2O4 cathode materials for Li-ion batteries. Journal of Power Sources, 2005, 150: 216-222. |
| [25] | SHEN C H, LIU R S, GUNDAKARAM R. Effect of Co doping in LiMn2O4. Journal of Power Sources, 2001, 102(1/2): 21-28. |
| [26] | SHANNON R D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallographica Section A, 1976, 32: 751-767. |
| [27] | GAO Y, REIMERS J N, DAHN J R. Changes in the voltage profile of Li/Li1+xMn2-xO4 cells as a function of x. Physical Review B, 1996, 54: 3878. |
| [28] | JAMES G S. Lange's Handbook of Chemistry. McGraw-Hill, New York, 1992. |
| [29] | KONG L, LI Y J, LI W J, et al. Synthesis and characterization of Li1.035Mn1.965O4 and Al-doped Li1.035Al0.035Mn1.930O4 as cathode materials for Li-ion batteries by a wet-chemical technique. Journal of Inorganic Materials, 2013, 28(3): 336-340. |
| [30] | LIANG Y Y, BAO S J, LI H L. A series of spinel phase cathode materials prepared by a simple hydrothermal process for rechargeable lithium batteries . Journal of Solid State Chemistry, 2006, 179(7): 2133-2140. |
| [31] | HJELM A K, LINDBERGH G. Experimental and theoretical analysis of LiMn2O4 cathodes for use in rechargeable lithium batteries by electrochemical impedance spectroscopy (EIS). Electrochimica Acta, 2002, 47(11): 1747-1759. |
| [32] | GUO Z P, ZHONG S, WANG G X. Structure and electrochemical characteristics of LiMn0.7M0.3O2(M=Ti, V, Zn, Mo, Co, Mg, Cr). Journal of Alloys and Compounds, 2003, 348(1/2): 231-235. |
| [33] | SHARMA Y, SHARMA N, SUBBA RAO G V. Lithium-storage and cycleability of nano-CdSnO3 as an anode material for lithium- ion batteries. Journal of Power Sources, 2009, 192(2): 627-635. |
| [1] | YAO Yishuai, GUO Ruihua, AN Shengli, ZHANG Jieyu, CHOU Kuochih, ZHANG Guofang, HUANG Yarong, PAN Gaofei. In-situ Loaded Pt-Co High Index Facets Catalysts: Preparation and Electrocatalytic Performance [J]. Journal of Inorganic Materials, 2023, 38(1): 71-78. |
| [2] | ZHANG Xian, ZHANG Ce, JIANG Wenjun, FENG Deqiang, YAO Wei. Synthesis, Electronic Structure and Visible Light Photocatalytic Performance of Quaternary BiMnVO5 [J]. Journal of Inorganic Materials, 2022, 37(1): 58-64. |
| [3] | XIAO Yumin, Li Bin, QIN Lizhao, LIN Hua, LI Qing, LIAO Bin. Efficient Preparation of CuGeO3 with Controllable Morphology Using CuCl2 as Copper Source [J]. Journal of Inorganic Materials, 2021, 36(1): 69-74. |
| [4] | WANG Juhan,WEN Xiong,LIU Chengchao,ZHANG Yuhua,ZHAO Yanxi,LI Jinlin. Preparation and Fischer-Tropsch Synthesis Performance of Hierarchical Co/Al-SiO2 Catalyst [J]. Journal of Inorganic Materials, 2020, 35(9): 999-1004. |
| [5] | XU Yun-Qing,WANG Hai-Zeng. Sodium Magnesium Fluoride Particles of Different Morphologies: Prepared by EDTA-assisted Hydrothermal Method [J]. Journal of Inorganic Materials, 2019, 34(9): 933-937. |
| [6] | GOU Sheng-Lian, NAI Xue-Ying, XIAO Jian-Fei, YE Jun-Wei, DONG Ya-Ping, LI Wu. Preparation and Thermal Decomposition of Basic Magnesium Chloride Whiskers [J]. Journal of Inorganic Materials, 2019, 34(7): 781-785. |
| [7] | Wei LIU, Kai ZHENG, Dong-Hong WANG, Yi-San LEI, Huai-Lin FAN. Co3O4 Nanowire Arrays@Activated Carbon Fiber Composite Materials: Facile Hydrothermal Synthesis and Its Electrochemical Application [J]. Journal of Inorganic Materials, 2019, 34(5): 487-492. |
| [8] | WANG Wei, LUO Shi-Jie, XIAN Cong, XIAO Qun, YANG Yang, OU Yun, LIU Yun-Ya, XIE Shu-Hong. Enhanced Thermoelectric Properties of Hydrothermal Synthesized BiCl3/Bi2S3 Composites [J]. Journal of Inorganic Materials, 2019, 34(3): 328-334. |
| [9] | JIANG Hai-Yan, XIA Yun-Sheng, LI Yu-Zhen. Preparation and Visible-light-driven Photocatalytic Performance of Porous Rod-like FeVO4 [J]. Journal of Inorganic Materials, 2018, 33(9): 949-955. |
| [10] | ZENG Yan-Fei, XIN Guo-Xiang, BULIN Chao-Ke, ZHANG Bang-Wen. One-step Preparation and Electrochemical Performance of 3D Reduced Graphene Oxide/NiO as Supercapacitor Electrodes Materials [J]. Journal of Inorganic Materials, 2018, 33(10): 1070-1076. |
| [11] | LI Guo-Chang, WANG Ping, LIU Chang-Bo. Hydrothermal Synthesis of Whitlockite [J]. Journal of Inorganic Materials, 2017, 32(11): 1128-1132. |
| [12] | MA Fang, CUI Ming-Fang, ZHU Jian-Hua, LI Ya-Li. Porous Hydroxyapatite Microspheres Prepared by Using Poly (Allylamine Hydrochloride) and Its Application in Drug Delivery [J]. Journal of Inorganic Materials, 2017, 32(11): 1215-1222. |
| [13] | RAN Hui-Li, HUANG Hao, MA Meng-Jun, ZHAI Jin-Sheng, FAN Jia-Jie. Dye-sensitized Solar Cells Based on Double-layer Composite Film with Enhanced Photovoltaic Performance [J]. Journal of Inorganic Materials, 2017, 32(10): 1049-1054. |
| [14] | CUI Lei, YANG Li-Juan, WANG Fan, XIA Wei-Wei. Fabrication of Flower-like Sn3O4 Hollow Microspheres and Their Photocatalytic Activity [J]. Journal of Inorganic Materials, 2016, 31(5): 461-465. |
| [15] | ZHAO De-Rui, ZHAI Ying-Jiao, LI Jin-Hua, CHU Xue-Ying, XU Ming-Ze, LI Xue, FANG Xuan, WEI Zhi-Peng, WANG Xiao-Hua. Preparation and Properties of Glucose Biosensor Based on Flower-like MoS2 Micrometer Material [J]. Journal of Inorganic Materials, 2016, 31(2): 153-158. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||