
Journal of Inorganic Materials ›› 2014, Vol. 29 ›› Issue (3): 269-274.DOI: 10.3724/SP.J.1077.2014.13275
• Orginal Article • Previous Articles Next Articles
ZHANG Yu-Hui1, YI Qing-Feng1, LIU Xiao-Ping1, XIANG Bai-Lin2
Received:2013-05-24
Revised:2013-07-03
Published:2014-03-20
Online:2014-02-18
About author:ZHANG Yu-Hui. E-mail:zhangyuhui110@126.com
CLC Number:
ZHANG Yu-Hui, YI Qing-Feng, LIU Xiao-Ping, XIANG Bai-Lin. Carbonizing Products of the Fe/Co Doped Polypyrrole as Efficient Electrocatalysts for Oxygen Reduction Reaction[J]. Journal of Inorganic Materials, 2014, 29(3): 269-274.
Add to citation manager EndNote|Ris|BibTeX
| Catalysts | PPY | PPY-Fe | PPY-Co | PPY-Fe-Co |
|---|---|---|---|---|
| Fe | 0 | 4.6wt% | 0 | 3.3wt% |
| Co | 0 | 0 | 3.8wt% | 2.7wt% |
Table1 Mass percentage of metal in catalyst
| Catalysts | PPY | PPY-Fe | PPY-Co | PPY-Fe-Co |
|---|---|---|---|---|
| Fe | 0 | 4.6wt% | 0 | 3.3wt% |
| Co | 0 | 0 | 3.8wt% | 2.7wt% |
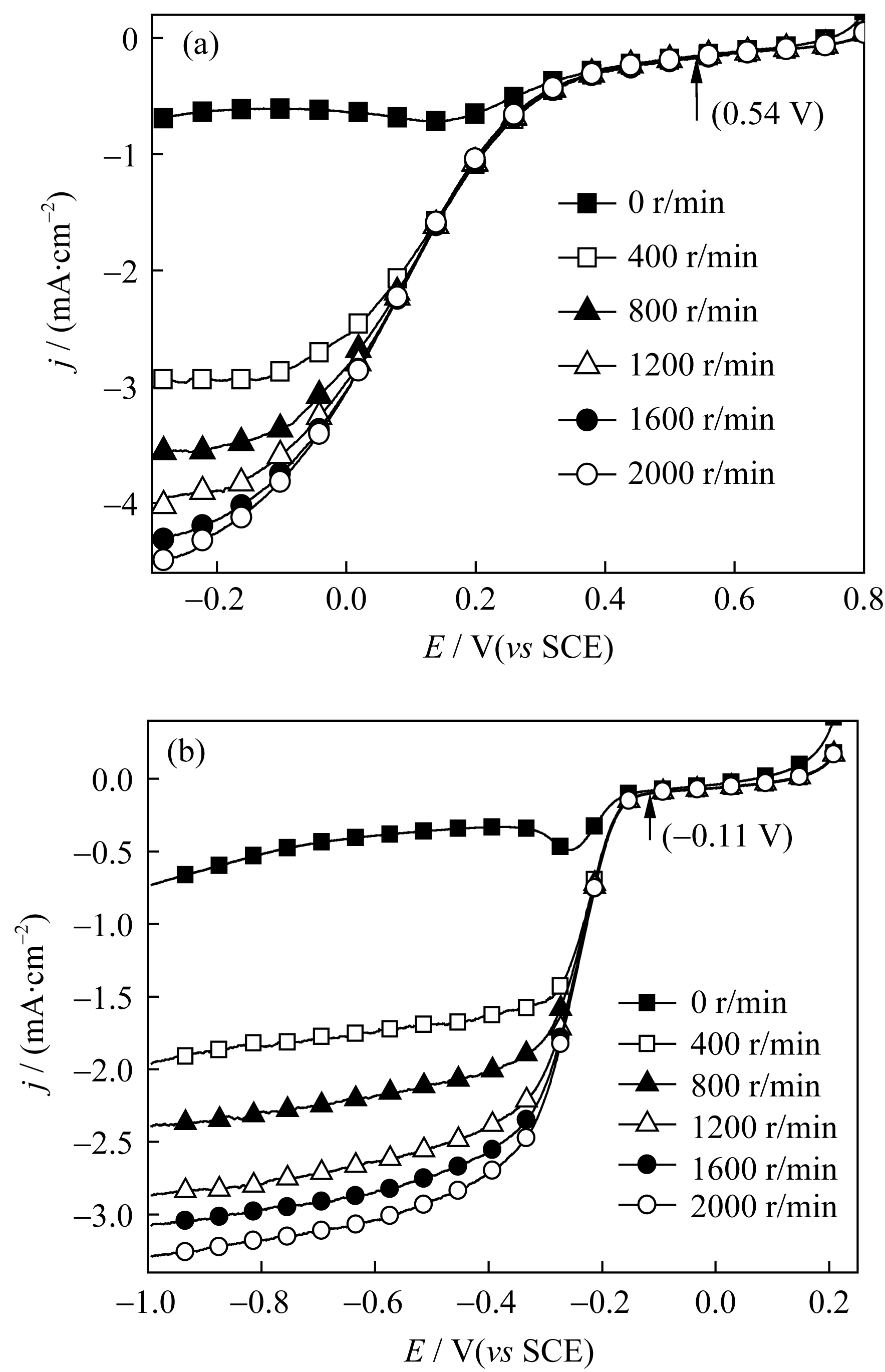
Fig. 7 RDE polarization curves of the PPY-Co at different rotation rates in O2-saturated 0.5 mol/L H2SO4 (a) and O2-saturated 1 mol/L NaOH (b). Scan rate at 5 mV/s
| Electrolyte | PPY | PPY-Fe | PPY-Co | PPY-Fe-Co |
|---|---|---|---|---|
| Acid | 1.8 | 2.4 | 3.4 | 2.5 |
| Alkaline | 1.5 | 2.7 | 3.2 | 2.1 |
Table 2 Electron-transfer number of ORR on various catalysts
| Electrolyte | PPY | PPY-Fe | PPY-Co | PPY-Fe-Co |
|---|---|---|---|---|
| Acid | 1.8 | 2.4 | 3.4 | 2.5 |
| Alkaline | 1.5 | 2.7 | 3.2 | 2.1 |
| [1] | JU JIAN-FENG, WU DONG-HUI. Development of anode catalysts for direct methanol fuel cell. Chemical Industry and Engineering Progress, 2009, 28(4): 646-649. |
| [2] | ANTOINE O, BULTEL Y, OZIL P, et al. Catalyst gradient for cathode active layer of proton exchange membrane fuel cell. Electrochim. Acta, 2000, 45(27): 4493-4500. |
| [3] | JIANG S P, LIN Z G, TSEUNG A C. Homogeneous and heterogeneous catalytic reactions in cobalt oxide/graphite air electrodes. Electrochem Soc., 1990, 137(3): 759-764. |
| [4] | LADOUCEUR M, LALANDE G, GUAY D, et al. Pyrolyzed cobalt phthalocyanine as electrocatalyst for oxygen reduction. Electrochem Soc., 1993, 140(7): 1974-1981. |
| [5] | THOMAS S C, REN X, GOTTESFELD S, et al. Direct methanol fuel cells: progress in cell performance and cathode research. Electrochim. Acta, 2002, 47(22/23): 3741-3748. |
| [6] | ASINSKI J. A new fuel cell cathode catalyst. Nature, 1964, 201:1212-1213. |
| [7] | ZAGAL J H, BEDIOUI F, DODELET J P. N4-Macrocyclic Metal Complexes. New York : Springer, 2006: 45. |
| [8] | SI YU-JUN, CHEN CHANG-GUO, XIONG ZHONG-PING, et al. Development of new type of carbon supported non-noble metal TM-N/C catalysts for oxygen reduction reaction. Chinese Journal of Power Sources, 2010, 34(12):1314-1317. |
| [9] | WU G, MORE K L, JOHNSTON C M, et al. High-performance electrocatalysts for oxygen reduction derived from polyaniline iron, and cobalt. Science, 2011, 332(6028): 443-447. |
| [10] | PER RUCHOT C, CHEHIMI M M. Alysis of conducting polypyrrole-silica gel composites. Synthetic Metal, 2000, 113: 53-63. |
| [11] | OMAST OVA M, SIMON F. Surface characterizations of conductive poly(methyl methacrylate)/polypyrrole composites. J. Mater. Sci., 2000, 35(7): 1743-1749. |
| [12] | SU WENCHENG, IROH IUDE O. Morphology and structure of the passive interphase formed during aqueous electrodeposition of polypyrrole coatings on steel. Electrochimica Acta, l999, 44(26): 4655-4665. |
| [13] | YUASA M, YAMAGUCHI A, ITSUKI H, et al. Modifying carbon particles with polypyrrole for adsorption of cobalt ions as electrocatatytic site for oxygen reduction. Chemistry Materials, 2005, 17(17): 4278-4281. |
| [14] | BASHYAM R, ZELENAY P. A class of non-precious metal composite catalysts for fuel cells. Nature, 2006, 443(7): 63-66. |
| [15] | LIU G, LI XG, GANESAN P, et al. Development of non-precious metal oxygen-reduction catalysts for PEM fuel cells based on N-doped ordered porous carbon. Applied Catalysis B: Environmental, 2009, 93(1/2): 156-165. |
| [16] | MATSUBARA K, WAKI K. The effect of O-Functionalities for the electrochemical reduction of oxygen on MWCNTS in acid media. Solid State Lett. 2010, 13(8): 7-9. |
| [17] | LIMA F H B, DE CASTRO J F R, TICIANELLI EDSON A. Silver-cobalt bimetallic particles for oxygen reduction in alkaline media. Journal of Power Sources, 2006,161(2): 806-812. |
| [18] | LIDE D R. CRC Handbook of Chemistry and Physics. CRC Press, Boca Raton, 2001. |
| [19] | LOBYNTSEVA E, KALLIO T, ALEXEYEVA N, et al. Electrochemical synthesis of hydrogen peroxide: Rotating disk electrode and fuel cell studies. Electrochimica Acta, 2007, 52(25): 7262-7269. |
| [1] | GUO Tianmin, DONG Jiangbo, CHEN Zhengpeng, RAO Mumin, LI Mingfei, LI Tian, LING Yihan. Enhanced Compatibility and Activity of High-entropy Double Perovskite Cathode Material for IT-SOFC [J]. Journal of Inorganic Materials, 2023, 38(6): 693-700. |
| [2] | YAO Yishuai, GUO Ruihua, AN Shengli, ZHANG Jieyu, CHOU Kuochih, ZHANG Guofang, HUANG Yarong, PAN Gaofei. In-situ Loaded Pt-Co High Index Facets Catalysts: Preparation and Electrocatalytic Performance [J]. Journal of Inorganic Materials, 2023, 38(1): 71-78. |
| [3] | SUN Lian, GU Quanchao, YANG Yaping, WANG Honglei, YU Jinshan, ZHOU Xingui. Two-dimensional Transition Metal Dichalcogenides for Electrocatalytic Oxygen Reduction Reaction [J]. Journal of Inorganic Materials, 2022, 37(7): 697-709. |
| [4] | JIANG Lili, XU Shuaishuai, XIA Baokai, CHEN Sheng, ZHU Junwu. Defect Engineering of Graphene Hybrid Catalysts for Oxygen Reduction Reactions [J]. Journal of Inorganic Materials, 2022, 37(2): 215-222. |
| [5] | FAN Shuai, JIN Tian, ZHANG Shanlin, LUO Xiaotao, LI Chengxin, LI Changjiu. Effect of Li2O Sintering Aid on Sintering Characteristics and Electrical Conductivity of LSGM Electrolyte for Solid Oxide Fuel Cell [J]. Journal of Inorganic Materials, 2022, 37(10): 1087-1092. |
| [6] | LIU Ziruo, LIU Wei, HAO Ce, HU Jinwen, SHI Yantao. Honeycomb-like Carbon-supported Fe Single Atom Catalyst: Preparation and Electrocatalytic Performance in Oxygen Reduction Reaction [J]. Journal of Inorganic Materials, 2021, 36(9): 943-949. |
| [7] | HAO Ce, LIU Ziruo, LIU Wei, SHI Yantao. Research Progress of Carbon-supported Metal Single Atom Catalysts for Oxygen Reduction Reaction [J]. Journal of Inorganic Materials, 2021, 36(8): 820-834. |
| [8] | ZHU Yong, GU Jun, YU Tao, HE Haitong, YAO Rui. Synthesis and Property of Platinum-cobalt Alloy Nano Catalyst [J]. Journal of Inorganic Materials, 2021, 36(3): 299-305. |
| [9] | CAO Dan,ZHOU Mingyang,LIU Zhijun,YAN Xiaomin,LIU Jiang. Fabrication and Characterization of Anode-supported Solid Oxide Fuel Cell Based on Proton Conductor Electrolyte [J]. Journal of Inorganic Materials, 2020, 35(9): 1047-1052. |
| [10] | DING Sheng, NING Kai, YUAN Binxia, PAN Weiguo, YIN Shibin, LIU Jianfeng. Durability of Fe-N/C Catalysts with Different Nanostructures for Electrochemical Oxygen Reduction in Alkaline Solution [J]. Journal of Inorganic Materials, 2020, 35(8): 953-958. |
| [11] | WU Fan, ZHAO Ziyan, LI Bangxin, DONG Fan, ZHOU Ying. Interfacial Oxygen Vacancy of Bi2O2CO3/PPy and its Visible-light Photocatalytic NO Oxidation Mechanism [J]. Journal of Inorganic Materials, 2020, 35(5): 541-548. |
| [12] | XIA Tian, MENG Xie, LUO Ting, ZHAN Zhongliang. La 3+-substituted Sr2Fe1.5Ni0.1Mo0.4O6-δ as Anodes for Solid Oxide Fuel Cells [J]. Journal of Inorganic Materials, 2020, 35(5): 617-622. |
| [13] | LUO Yi,FENG Junzong,FENG Jian,JIANG Yonggang,LI Liangjun. Research Progress on Advanced Carbon Materials as Pt Support for Proton Exchange Membrane Fuel Cells [J]. Journal of Inorganic Materials, 2020, 35(4): 407-415. |
| [14] | LI Ya-Hui, ZHANG Jian-Feng, CAO Hui-Yang, ZHANG Xin, JIANG Wan. PtRu Particles Supported on Two-dimensional Titanium Carbide/Carbon Nanotubes: Preparation and Electrocatalytic Properties [J]. Journal of Inorganic Materials, 2020, 35(1): 79-85. |
| [15] | Kai LI, Xiao LI, Jian LI, Jia-Miao XIE. Structural Stability of Ni-Fe Supported Solid Oxide Fuel Cells Based on Stress Analysis [J]. Journal of Inorganic Materials, 2019, 34(6): 611-617. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||