
Journal of Inorganic Materials ›› 2012, Vol. 27 ›› Issue (2): 185-190.DOI: 10.3724/SP.J.1077.2012.00185
• Orginal Article • Previous Articles Next Articles
WANG Fang, LI Jian-Sheng, RAN Dong-Qin, DUAN Meng-Shan, SUN Xiu-Yun, WANG Lian-Jun
Received:2011-01-17
Revised:2011-03-11
Published:2012-02-10
Online:2012-01-05
About author:WANG Fang. E-mail: su_daily@126.com
Supported by:CLC Number:
WANG Fang, LI Jian-Sheng, RAN Dong-Qin, DUAN Meng-Shan, SUN Xiu-Yun, WANG Lian-Jun. Active Biocatalysts Based on Pepsin Immobilized in Short Channeled Zr-Ce-SBA-15[J]. Journal of Inorganic Materials, 2012, 27(2): 185-190.
Add to citation manager EndNote|Ris|BibTeX
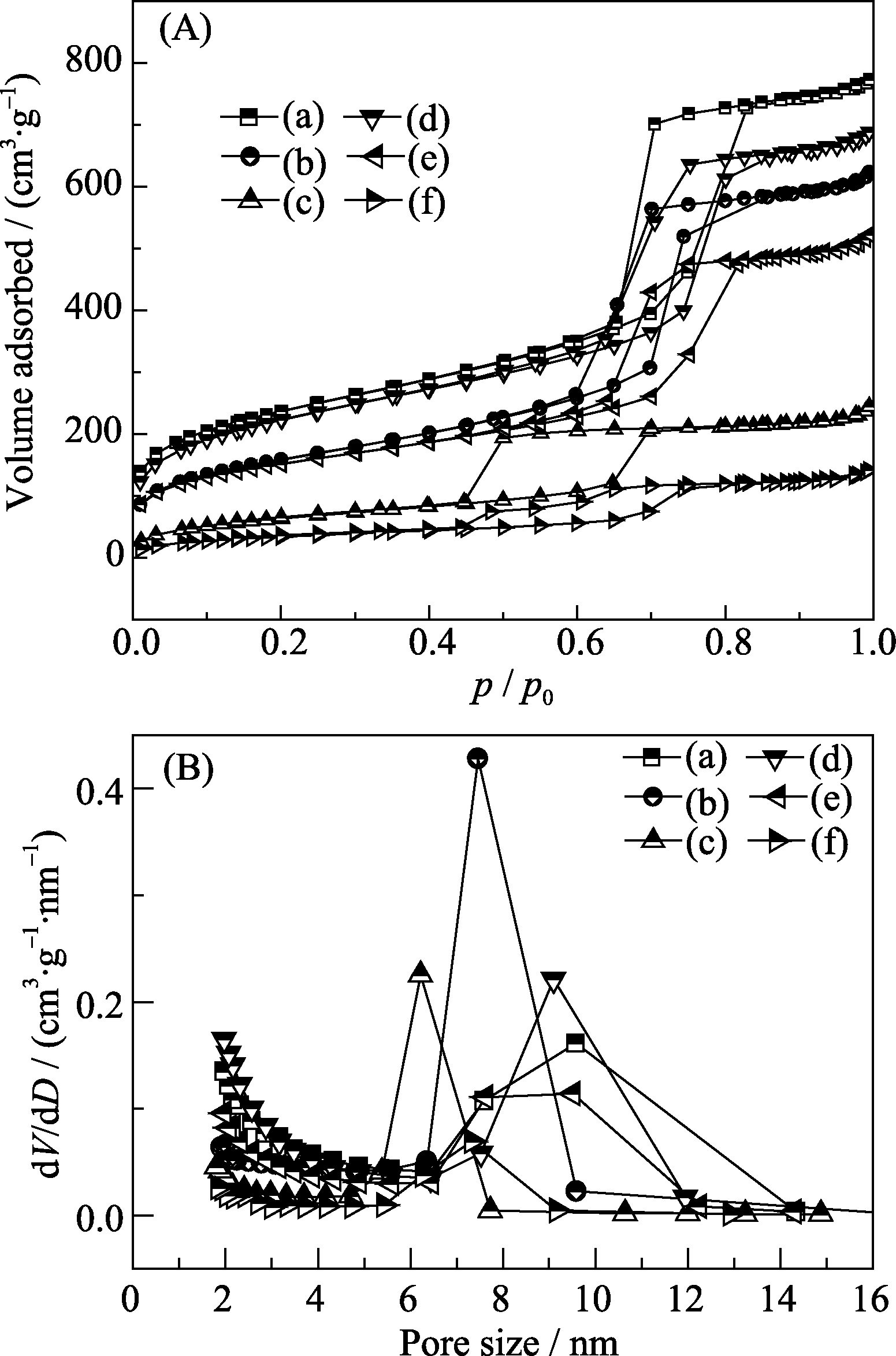
Fig. 3 N2 adsorption-desorption isotherms (A) and pore size distributions (B) of SBA-15 (a), PEP/SBA-15 (b), PEP/ SBA-15/AAPTS (c), ZCS (d), PEP/ZCS (e) and PEP/ZCS/ AAPTS (f)
| Sample | SBET/(m2·g-1) | D/nm | V/(cm3·g-1) |
|---|---|---|---|
| SBA-15 | 847 | 9.6 | 1.16 |
| PEP/SBA-15 | 577 | 7.5 | 0.99 |
| PEP/SBA-15/AAPTS | 241 | 6.2 | 0.39 |
| ZCS | 805 | 9.2 | 1.03 |
| PEP/ZCS | 547 | 8.4 | 0.80 |
| PEP/ZCS/AAPTS | 129 | 7.3 | 0.22 |
Table 1 Textural properties of samples
| Sample | SBET/(m2·g-1) | D/nm | V/(cm3·g-1) |
|---|---|---|---|
| SBA-15 | 847 | 9.6 | 1.16 |
| PEP/SBA-15 | 577 | 7.5 | 0.99 |
| PEP/SBA-15/AAPTS | 241 | 6.2 | 0.39 |
| ZCS | 805 | 9.2 | 1.03 |
| PEP/ZCS | 547 | 8.4 | 0.80 |
| PEP/ZCS/AAPTS | 129 | 7.3 | 0.22 |
| Sample | Relative enzyme leaching amount/% |
|---|---|
| PEP/SBA-15 | 20.7 |
| PEP/ZCS | 21.4 |
| PEP/SBA-15/AAPTS | 7.7 |
| PEP/ZCS/AAPTS | 7.2 |
Table 2 Leakage of adsorbed enzyme
| Sample | Relative enzyme leaching amount/% |
|---|---|
| PEP/SBA-15 | 20.7 |
| PEP/ZCS | 21.4 |
| PEP/SBA-15/AAPTS | 7.7 |
| PEP/ZCS/AAPTS | 7.2 |
| Sample | Enzyme activity / (U·mg-1) | Relative enzyme activity/% | ||
|---|---|---|---|---|
| Initial | After 4 cycles | Initial | After 4 cycles | |
| Free pepsin | 28.71 | — | 100% | — |
| PEP/SBA-15 | 7.34 | 3.06 | 25.6% | 10.6% |
| PEP/ZCS | 9.65 | 2.76 | 33.6% | 9.6% |
| PEP/SBA-15/AAPTS | 4.88 | 4.75 | 17.0% | 16.5% |
| PEP/ZCS/AAPTS | 6.23 | 6.00 | 21.7% | 20.9% |
Table 3 Catalytic activity test for peptic hydrolysis
| Sample | Enzyme activity / (U·mg-1) | Relative enzyme activity/% | ||
|---|---|---|---|---|
| Initial | After 4 cycles | Initial | After 4 cycles | |
| Free pepsin | 28.71 | — | 100% | — |
| PEP/SBA-15 | 7.34 | 3.06 | 25.6% | 10.6% |
| PEP/ZCS | 9.65 | 2.76 | 33.6% | 9.6% |
| PEP/SBA-15/AAPTS | 4.88 | 4.75 | 17.0% | 16.5% |
| PEP/ZCS/AAPTS | 6.23 | 6.00 | 21.7% | 20.9% |
| [1] | Hartmann M. Ordered mesoporous materials for bioadsorption and biocatalysis. Chem. Mater., 2005, 17(18): 4577-4593. |
| [2] | 胡 燚, 刘维明, 邹 彬, 等. 介孔材料固定化酶. 化学进展, 2010, 22(8): 1656-1664. |
| [3] | Wang Y J, Caruso F. Mesoporous silica spheres as supports for enzyme immobilization and encapsulation. Chem. Mater., 2005, 17(5): 953-961. |
| [4] | Fan J, Wang L M, Yu C Z, et al. Rapid and high-capacity immobilization of enzymes based on mesoporous silicas with controlled morphologies. Chem. Commun., 2003, (17): 2140-2141. |
| [5] | Salis A, Meloni D, Ligas S, et al. Physical and chemical adsorption of mucor javanicus lipase on SBA-15 mesoporous silica. synthesis, structural characterization, and activity performance. Langmuir, 2005, 21(12): 5511-5516. |
| [6] | 高 波, 朱广山, 傅学奇, 等(GAO, et al). 介孔分子筛SBA-15 中α-胰凝乳蛋白酶组装及催化活性研究. 高等学校化学学报(Chem. J. Chinese U.), 2003, 24(6): 1100-1102. |
| [7] | Schlossbauer A, Schaffert D, Kecht J, et al. Click chemistry for high-density biofunctionalization of mesoporous silica. J. Am. Chem. Soc., 2008, 130(38): 12558-12559. |
| [8] | Chen S Y, Tang C Y, Chuang W T, et al. A facile route to synthesizing functionalized mesoporous SBA-15 materials with platelet morphology and short mesochannels. Chem. Mater., 2008, 20(12): 3906-3916. |
| [9] | Li Y J, Zhou G W, Qiao W T, et al. Immobilization of porcine pancreas lipase on fiber-like SBA-15 mesoporous material. Mater. Sci. Eng., B, 2009, 162(2): 120-126. |
| [10] | Shah P, Sridevi N, Prabhune A, et al. Structural features of penicillin acylase adsorption on APTES functionalized SBA-15. Microporous Mesoporous Mater., 2008, 116(1/2/3): 157-165. |
| [11] | Lei J, Fan J, Yu C, et al. Immobilization of enzymes in mesoporous materials: controlling the entrance to nanospace. Microporous Mesoporous Mater. , 2004, 73(3): 121-128. |
| [12] | 袁金芳, 李健生, 顾 娟, 等(YUAN Jin-Fang, et al). 一种合成六方板状Zr-Ce-SBA-15介孔材料的新方法. 化学学报(Acta Chim. Sinica), 2009, 67(11): 1271-1275. |
| [13] | Zhao D Y, Feng J L, Huo Q S, et al. Triblock copolymer syntheses of mesoporous silica with periodic 50 to 300 angstrom pores. Science, 1998, 279(5350): 548-552. |
| [14] | Manyar H G, Gianotti E, Sakamoto Y, et al. Active biocatalysts based on pepsin immobilized in mesoporous SBA-15. J. Phys. Chem. C, 2008, 112(46): 18110-18116. |
| [15] | Zhao D Y, Sun J Y, Li Q Z, et al. Morphological control of highly ordered large pore mesoporous silica SBA -15. Chem. Mater., 2000, 12(2): 275-279. |
| [16] | Prasetyanto E A, Park S E. Synthesis of short-channeled amino-functionalized SBA-15 and its beneficial applications in base- catalyzed reactions. Appl. Catal. A: Gen., 2008, 350(2): 244-251. |
| [17] | 李健生, 苗小郁, 易 睿, 等. 巯基功能化介孔氧化硅的合成及其对Pb(Ⅱ)的吸附. 功能材料, 2007, 38(8): 1386-1388. |
| [18] | Oberg K A, Fink A L. A new attenuated total reflectance Fourier transform infrared spectroscopy method for the study of proteins in solution. Anal. Biochem., 1998, 256(1): 92-106. |
| [19] | Yang L, Shen Q, Zhou J, et al. Biomimetic synthesis of CdS nanocrystals in aqueous solution of pepsin. Mater. Chem. Phys., 2006, 98(1): 125-130. |
| [20] | Seo Y K, Park S B, Park D H, et al. Mesoporous hybrid organosilica containing urethane moieties. J. Sol. State. Chem., 2006, 179(4): 1285-1288. |
| [21] | Sun J. M, Zhang H, Tian R J, et al. Ultrafast enzyme immobilization over large-pore nanoscale mesoporous silica particles. Chem. Commun., 2006, (12): 1322-1324. |
| [22] | Gao Q, Xu W, Xu Y, et al. Amino acid adsorption on mesoporous materials: influence of types of amino acids, modification of mesoporous materials, and solution conditions. J. Phys. Chem. B, 2008, 112(7): 2261-2267. |
| [23] | Hudson S, Magner E, Cooney J, et al. Methodology for the immobilization of enzymes onto mesoporous materials. J. Phys. Chem. B, 2005, 109(41): 19496-19506. |
| [24] | Liu Y G, Xu Q, Feng X M, et al. Immobilization of hemoglobin on SBA-15 applied to the electrocatalytic reduction of H2O2. Anal. Bioanal. Chem., 2007, 387(4): 1553-1559. |
| [1] | WU Lin, HU Minglei, WANG Liping, HUANG Shaomeng, ZHOU Xiangyuan. Preparation of TiHAP@g-C3N4 Heterojunction and Photocatalytic Degradation of Methyl Orange [J]. Journal of Inorganic Materials, 2023, 38(5): 503-510. |
| [2] | MA Xinquan, LI Xibao, CHEN Zhi, FENG Zhijun, HUANG Juntong. BiOBr/ZnMoO4 Step-scheme Heterojunction: Construction and Photocatalytic Degradation Properties [J]. Journal of Inorganic Materials, 2023, 38(1): 62-70. |
| [3] | CHEN Hanxiang, ZHOU Min, MO Zhao, YI Jianjian, LI Huaming, XU Hui. 0D/2D CoN/g-C3N4 Composites: Structure and Photocatalytic Performance for Hydrogen Production [J]. Journal of Inorganic Materials, 2022, 37(9): 1001-1008. |
| [4] | HU Yue, AN Lin, HAN Xin, HOU Chengyi, WANG Hongzhi, LI Yaogang, ZHANG Qinghong. RhO2 Modified BiVO4 Thin Film Photoanodes: Preparation and Photoelectrocatalytic Water Splitting Performance [J]. Journal of Inorganic Materials, 2022, 37(8): 873-882. |
| [5] | XUE Hongyun, WANG Congyu, MAHMOOD Asad, YU Jiajun, WANG Yan, XIE Xiaofeng, SUN Jing. Two-dimensional g-C3N4 Compositing with Ag-TiO2 as Deactivation Resistant Photocatalyst for Degradation of Gaseous Acetaldehyde [J]. Journal of Inorganic Materials, 2022, 37(8): 865-872. |
| [6] | SUN Lian, GU Quanchao, YANG Yaping, WANG Honglei, YU Jinshan, ZHOU Xingui. Two-dimensional Transition Metal Dichalcogenides for Electrocatalytic Oxygen Reduction Reaction [J]. Journal of Inorganic Materials, 2022, 37(7): 697-709. |
| [7] | CHI Congcong, QU Panpan, REN Chaonan, XU Xin, BAI Feifei, ZHANG Danjie. Preparation of SiO2@Ag@SiO2@TiO2 Core-shell Structure and Its Photocatalytic Degradation Property [J]. Journal of Inorganic Materials, 2022, 37(7): 750-756. |
| [8] | WANG Xiaojun, XU Wen, LIU Runlu, PAN Hui, ZHU Shenmin. Preparation and Properties of Ag@C3N4 Photocatalyst Supported by Hydrogel [J]. Journal of Inorganic Materials, 2022, 37(7): 731-740. |
| [9] | FU Yongsheng, BI Min, LI Chun, SUN Jingwen, WANG Xin, ZHU Junwu. Research Progress on Non-noble Metal/Nitrogen-doped Carbon Composite Materials in Electrocatalytic Oxygen Evolution Reaction [J]. Journal of Inorganic Materials, 2022, 37(2): 163-172. |
| [10] | WU Jing, YU Libing, LIU Shuaishuai, HUANG Qiuyan, JIANG Shanshan, ANTON Matveev, WANG Lianli, SONG Erhong, XIAO Beibei. NiN4/Cr Embedded Graphene for Electrochemical Nitrogen Fixation [J]. Journal of Inorganic Materials, 2022, 37(10): 1141-1148. |
| [11] | LIU Xuechen, ZENG Di, ZHOU Yuanyi, WANG Haipeng, ZHANG Ling, WANG Wenzhong. Selective Oxidation of Biomass over Modified Carbon Nitride Photocatalysts [J]. Journal of Inorganic Materials, 2022, 37(1): 38-44. |
| [12] | ZHANG Xian, ZHANG Ce, JIANG Wenjun, FENG Deqiang, YAO Wei. Synthesis, Electronic Structure and Visible Light Photocatalytic Performance of Quaternary BiMnVO5 [J]. Journal of Inorganic Materials, 2022, 37(1): 58-64. |
| [13] | WANG Xiao, ZHU Zhijie, WU Zhiyi, ZHANG Chengcheng, CHEN Zhijie, XIAO Mengqi, LI Chaoran, HE Le. Preparation and Photothermal Catalytic Application of Powder-form Cobalt Plasmonic Superstructures [J]. Journal of Inorganic Materials, 2022, 37(1): 22-28. |
| [14] | LIU Peng, WU Shimiao, WU Yunfeng, ZHANG Ning. Synthesis of Zn0.4(CuGa)0.3Ga2S4/CdS Photocatalyst for CO2 Reduction [J]. Journal of Inorganic Materials, 2022, 37(1): 15-21. |
| [15] | WANG Luping, LU Zhanhui, WEI Xin, FANG Ming, WANG Xiangke. Application of Improved Grey Model in Photocatalytic Data Prediction [J]. Journal of Inorganic Materials, 2021, 36(8): 871-876. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||