
Journal of Inorganic Materials ›› 2012, Vol. 27 ›› Issue (2): 139-145.DOI: 10.3724/SP.J.1077.2012.00139
• Orginal Article • Previous Articles Next Articles
XU Dong1,2, ZHANG Jun2, LI Gang2, XIAO Penny2, WEBLEY Paul2, ZHAI Yu-Chun1
Received:2011-01-13
Revised:2011-03-04
Published:2012-02-10
Online:2012-01-05
About author:XU Dong.E-mail: e3e4sun@gmail.com
CLC Number:
XU Dong, ZHANG Jun, LI Gang, XIAO Penny, WEBLEY Paul, ZHAI Yu-Chun. Adsorption Equilibrium and Kinetics of CO2 and H2O on ActivatedCarbon[J]. Journal of Inorganic Materials, 2012, 27(2): 139-145.
Add to citation manager EndNote|Ris|BibTeX
| Property | Activated carbon |
|---|---|
| Original material | Coconut shell |
| Shape | Granular |
| Activated method | Steam |
| BET surface/(m2·g-1) | 921.7 |
| Total pore volume /(cm3·g-1) | 0.37 |
| Nominal pore size /nm | 0.73 |
Table 1 Physical properties of activated carbon
| Property | Activated carbon |
|---|---|
| Original material | Coconut shell |
| Shape | Granular |
| Activated method | Steam |
| BET surface/(m2·g-1) | 921.7 |
| Total pore volume /(cm3·g-1) | 0.37 |
| Nominal pore size /nm | 0.73 |
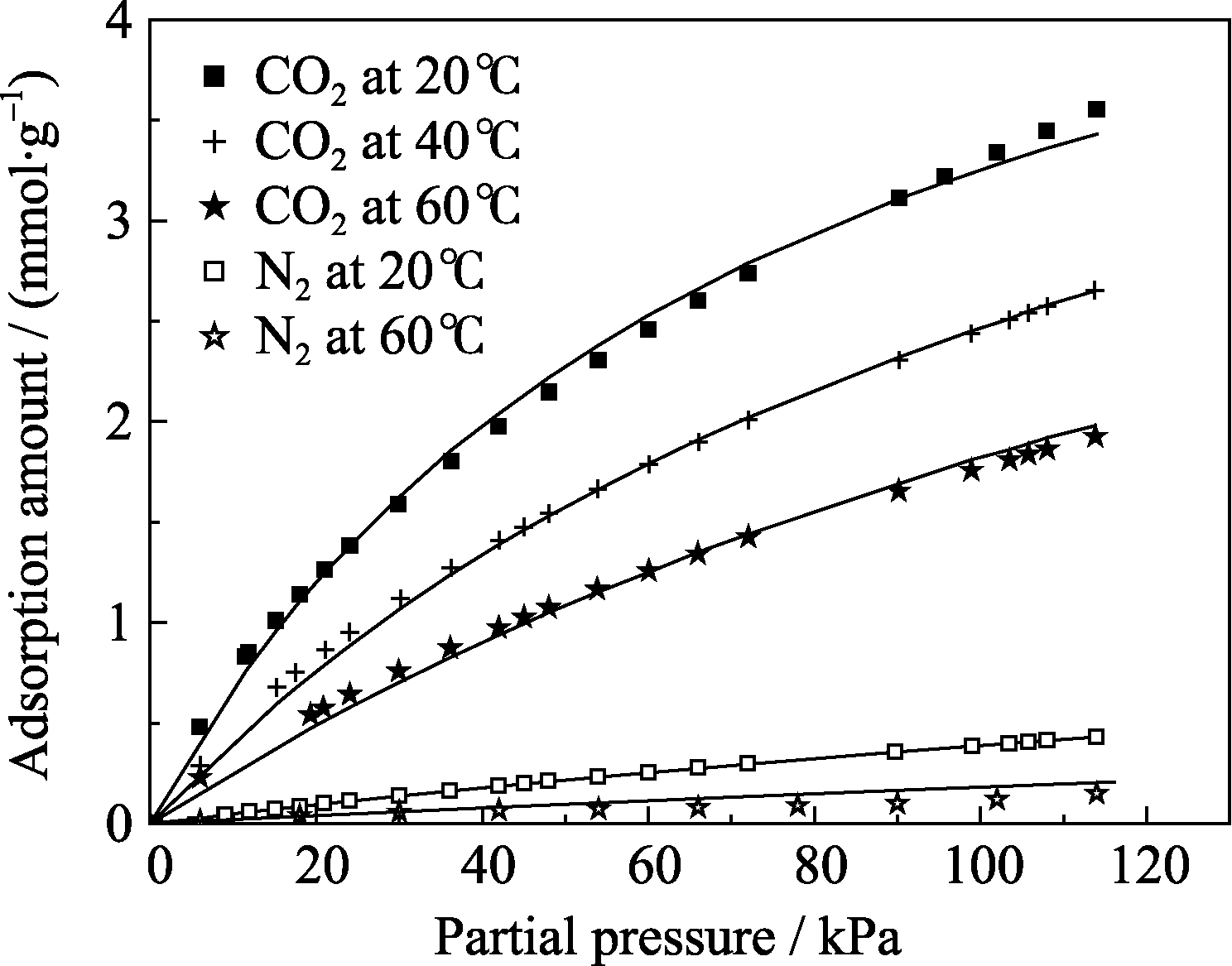
Fig. 6 Adsorption isotherms of CO2 and N2 onto activated carbon Dots are experimental results and lines represent model fitting by single site Langmuir equation
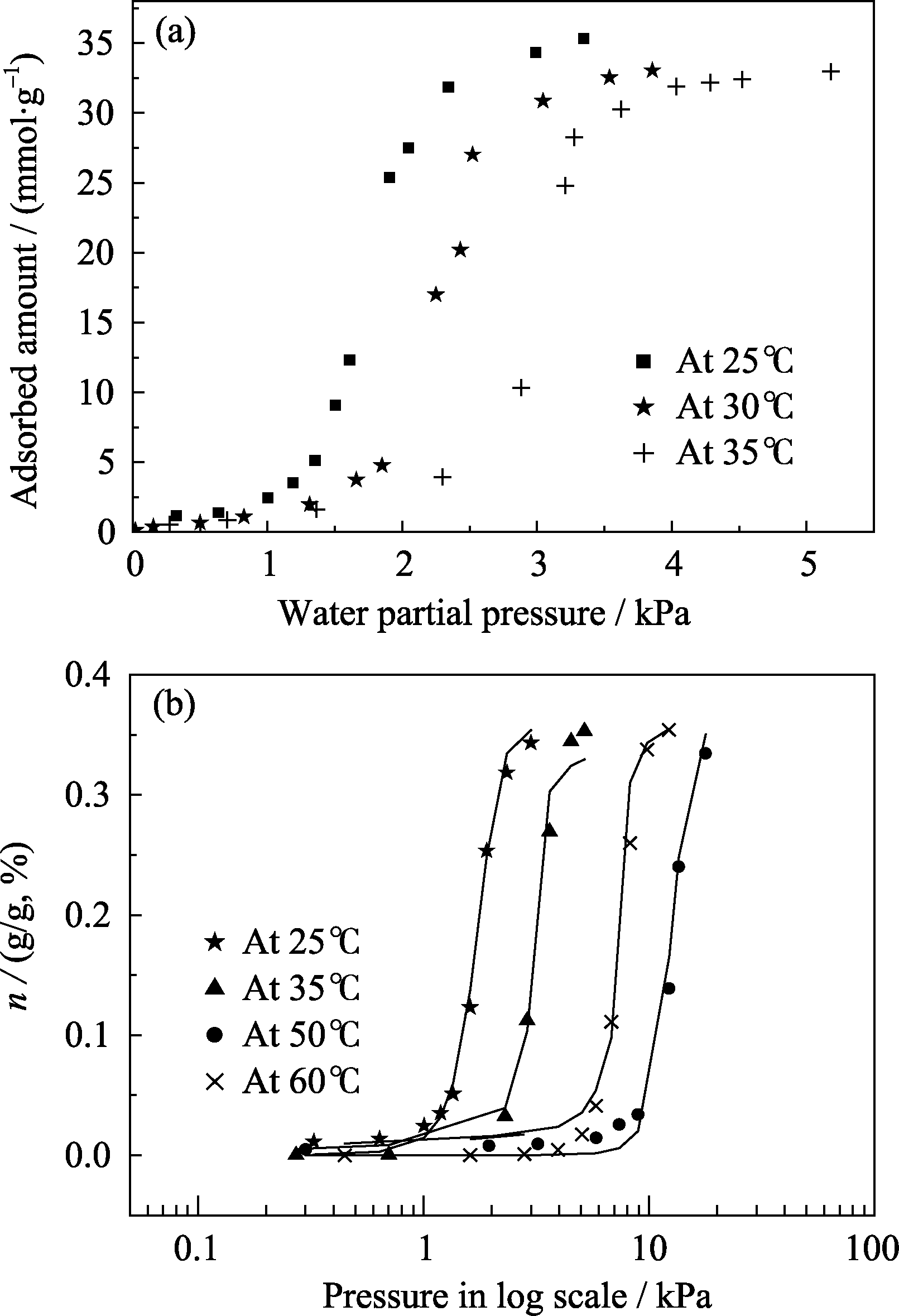
Fig. 7 Adsorption isotherms of water vapor onto activated carbon at (a) 25, 30 and 35℃ of experimental data, (b) 25, 35, 50 and 60℃ with model fitting data
| [1] | 费维扬, 艾 宁, 陈 健. 温室气体的捕集和分离-分离技术面临的挑战与机遇. 化工进展, 2005, 24(1):1-4. |
| [2] | Jose D F, TIimothy F, Sean P, et al. Advances in CO2 capture technology—The U.S. department of energy’s carbon sequestration program. Journal of Greenhouse Gas Control, 2008, 2(1): 9-20. |
| [3] | 李天成, 冯 霞, 李鑫钢. 二氧化碳处理技术现状及其发展趋势. 化学工业与工程, 2002, 19(2):190-197. |
| [4] | Xu X, Wang D. Reducing greenhouse gas emissions from energy consumption activities by the iron and steel industry in East China. Energy Sources, 1999, 21(6): 541-546. |
| [5] | Yang R T. Gas Separation by Adsorption Processes. Michigan: Imperial Collage Press, 1997: 237-247. |
| [6] | Takamura Y S, Narita J, Aoki S. Evaluation of dual-bed pressure swing adsorption for CO2 recovery from boiler exhaust gas. Separation and Purification Technology, 2001, 24(3): 519-522. |
| [7] | Daeho K, Ranjani S, Lorenz T B. Optimization of pressure swing adsorption and fractionated vacuum pressure swing adsorption processes for CO2 capture. Ind. Eng. Chem. Res., 2005, 44(21): 8084-8094. |
| [8] | Zhang J, Xiao P, Li G, et al. Effect of flue gas impurities on CO2 capture performance from flue gas at coal-fired power stations by vacuum swing adsorption. Energy Procedia, 2009, 1(1): 1115-1122. |
| [9] | Chue K T, Kim J N, Yoo Y J, et al. Comparison of activated carbon and zeolite 13X for CO2 recovery from flue gas by pressure swing adsorption. Ind. Eng. Chem. Res., 1995, 34(2): 591-598. |
| [10] | Xiao P, Zhang J, Webley P, et al. Capture of CO2 from flue gas streams with zeolite 13X by vacuum-pressure swing adsorption. Adsorption, 2008, 14(4/5): 575-582. |
| [11] | Harlick P J, Tezel F H. An experimental adsorbent screening study for CO2 removal from N2. Microporous and Mesoporous Materials, 2004, 76(1/2/3): 71-79. |
| [12] | Li G, Xiao P, Webley P, et al. Capture of CO2 from high humidity flue gas by vacuum swing adsorption with zeolite 13X. Adsorption, 2008, 14(2/3): 415-422. |
| [13] | Li G, Xiao P, Webley P, et al. Competition of CO2/H2O in adsorption based CO2 capture. Energy Procedia, 2009, 1(1): 1123-1130. |
| [14] | Ahn H, Lee C. Effects of capillary condensation on adsorption and thermal desorption dynamics of water in zeolite 13X and layered beds. Chemical Engineering Science, 2004, 59(13): 2727-2743. |
| [15] | 李 明, 周 理, 吴 琴. 多组分气体吸附平衡理论研究进展. 化学进展, 2002, 14(2): 93-98. |
| [16] | Qi S, Hay K J, Rood J M, et al. Equilibrium and heat of adsorption for water vapor and activated carbon. Journal of Environmental Engineering, 2000, 126(3): 267-271. |
| [17] | Rutherford S W. Modeling water adsorption in carbon micropores: study of water in carbon molecular sieves. Langmuir, 2006, 22(2): 702-708. |
| [18] | Mu1ller E A, Rull L F, Vega L F, et al. Adsorption of water on activated carbons: a molecular simulation study. J. Phys. Chem., 1996, 100(4): 1189-1196. |
| [19] | Murdock J N, Wetzel D L. FT-IR microspectroscopy enhances biological and ecological analysis of algae. Applied Spectroscopy Reviews, 2009, 44(4): 335-361. |
| [20] | McCallum C L, Bandosz T J, McGrothe S C, et al. A molecular model for adsorption of water on activated carbon: comparison of simulation and experiment. Langmuir, 1999, 15(2): 533-544. |
| [21] | CHEN Zhan-Ying, WANG Xu-Hui, WANG Ya-Long, et al. Dynamic adsorption and desorption properties of xenon on activated carbon fiber. Journal of Inorganic Materials, 2006, 21(1): 81-86. |
| [1] | MA Xiaosen, ZHANG Lichen, LIU Yanchao, WANG Quanhua, ZHENG Jiajun, LI Ruifeng. 13X@SiO2: Synthesis and Toluene Adsorption [J]. Journal of Inorganic Materials, 2023, 38(5): 537-543. |
| [2] | GUO Chunxia, CHEN Weidong, YAN Shufang, ZHAO Xueping, YANG Ao, MA Wen. Adsorption of Arsenate in Water by Zirconia-halloysite Nanotube Material [J]. Journal of Inorganic Materials, 2023, 38(5): 529-536. |
| [3] | WANG Shiyi, FENG Aihu, LI Xiaoyan, YU Yun. Pb (II) Adsorption Process of Fe3O4 Supported Ti3C2Tx [J]. Journal of Inorganic Materials, 2023, 38(5): 521-528. |
| [4] | YU Yefan, XU Ling, NI Zhongbing, SHI Dongjian, CHEN Mingqing. Prussian Blue Modified Biochar: Preparation and Adsorption of Ammonia Nitrogen from Sewage [J]. Journal of Inorganic Materials, 2023, 38(2): 205-212. |
| [5] | WANG Hongning, HUANG Li, QING Jiang, MA Tengzhou, HUANG Weiqiu, CHEN Ruoyu. Mesoporous Organic-inorganic Hybrid Siliceous Hollow Spheres: Synthesis and VOCs Adsorption [J]. Journal of Inorganic Materials, 2022, 37(9): 991-1000. |
| [6] | FENG Qingying, LIU Dong, ZHANG Ying, FENG Hao, LI Qiang. Thermodynamic and First-principles Assessments of Materials for Solar-driven CO2 Splitting Using Two-step Thermochemical Cycles [J]. Journal of Inorganic Materials, 2022, 37(2): 223-229. |
| [7] | LIU Cheng, ZHAO Qian, MOU Zhiwei, LEI Jiehong, DUAN Tao. Adsorption Properties of Novel Bismuth-based SiOCNF Composite Membrane for Radioactive Gaseous Iodine [J]. Journal of Inorganic Materials, 2022, 37(10): 1043-1050. |
| [8] | WANG Xiao, ZHU Zhijie, WU Zhiyi, ZHANG Chengcheng, CHEN Zhijie, XIAO Mengqi, LI Chaoran, HE Le. Preparation and Photothermal Catalytic Application of Powder-form Cobalt Plasmonic Superstructures [J]. Journal of Inorganic Materials, 2022, 37(1): 22-28. |
| [9] | LIU Peng, WU Shimiao, WU Yunfeng, ZHANG Ning. Synthesis of Zn0.4(CuGa)0.3Ga2S4/CdS Photocatalyst for CO2 Reduction [J]. Journal of Inorganic Materials, 2022, 37(1): 15-21. |
| [10] | ZHOU Fan, BI Hui, HUANG Fuqiang. Ultra-large Specific Surface Area Activated Carbon Synthesized from Rice Husk with High Adsorption Capacity for Methylene Blue [J]. Journal of Inorganic Materials, 2021, 36(8): 893-903. |
| [11] | YU Xiangkun, LIU Kun, LI Zhipeng, ZHAO Yulu, SHEN Jinyou, MAO Ping, SUN Aiwu, JIANG Jinlong. Efficient Adsorption of Radioactive Iodide by Copper/Palygorskite Composite [J]. Journal of Inorganic Materials, 2021, 36(8): 856-864. |
| [12] | SU Li, YANG Jianping, LAN Yue, WANG Lianjun, JIANG Wan. Interface Design of Iron Nanoparticles for Environmental Remediation [J]. Journal of Inorganic Materials, 2021, 36(6): 561-569. |
| [13] | XI Wen, LI Haibo. Preparation of TiO2/Ti3C2Tx Composite for Hybrid Capacitive Deionization [J]. Journal of Inorganic Materials, 2021, 36(3): 283-291. |
| [14] | WANG Tingting, SHI Shumei, LIU Chenyuan, ZHU Wancheng, ZHANG Heng. Synthesis of Hierarchical Porous Nickel Phyllosilicate Microspheres as Efficient Adsorbents for Removal of Basic Fuchsin [J]. Journal of Inorganic Materials, 2021, 36(12): 1330-1336. |
| [15] | GUO Yu, JIANG Xiaoqing, WU Hongmei, XIAO Yu, WU Dafu, LIU Xin. Preparation of 2-hydroxy-1-naphthalene Functionalized SBA-15 Adsorbent for the Adsorption of Chromium(III) Ions from Aqueous Solution [J]. Journal of Inorganic Materials, 2021, 36(11): 1163-1170. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||