无机材料学报 ›› 2023, Vol. 38 ›› Issue (11): 1309-1315.DOI: 10.15541/jim20230277
所属专题: 【能源环境】燃料电池(202312)
收稿日期:2023-06-12
修回日期:2023-07-21
出版日期:2023-08-21
网络出版日期:2023-08-21
作者简介:杨代辉(1993-), 男, 硕士. E-mail: 841671731@qq.com
基金资助:
YANG Daihui1( ), SUN Tian2, TIAN Hexin1, SHI Xiaofei1, MA Dongwei1
), SUN Tian2, TIAN Hexin1, SHI Xiaofei1, MA Dongwei1
Received:2023-06-12
Revised:2023-07-21
Published:2023-08-21
Online:2023-08-21
About author:YANG Daihui (1993-), male, Master. E-mail: 841671731@qq.com
Supported by:摘要:
氧还原反应(ORR)是燃料电池阴极重要的电化学反应过程, 其自发反应进程缓慢, 对氧还原反应起高效催化作用的催化剂面临价格昂贵、合成流程复杂、污染环境等问题, 因此探索合成简单、环境友好的氧还原催化剂制备方法具有重要意义。铁氮共掺杂介孔碳材料(Fe-N/MC)是一种有巨大应用价值的非贵金属氧还原反应催化剂。本工作通过在马弗炉中的半封闭体系内高温碳化小分子前驱体得到介孔碳材料(MCM), 再把获得的MCM与铁盐混合在管式炉中高温处理制备得到铁氮共掺杂介孔碳材料(Fe-N/MCMT)。该方法热解条件简单, 无需模板剂和NH3、HF等有毒物质。由于MCM含有较高的氮和氧元素, 有利于提升介孔碳材料表面的亲水性和配位能力, 通过MCM和铁盐制备出的Fe-N/MCMT含有丰富的、催化ORR的Fe-Nx活性位点, 其起始电位和半波电位分别为0.941和0.831 V (vs RHE), 比商业化Pt/C催化剂的起始电位和半波电位分别正34和16 mV。氧还原反应按照反应过程分为二电子过程和四电子过程, Fe-N/MCMT和Pt/C的转移电子数分别为3.77和3.91, 表明具有四电子反应过程。
中图分类号:
杨代辉, 孙甜, 田合鑫, 史晓斐, 马东伟. 铁氮共掺杂介孔碳材料的简易制备及其氧还原反应催化性能[J]. 无机材料学报, 2023, 38(11): 1309-1315.
YANG Daihui, SUN Tian, TIAN Hexin, SHI Xiaofei, MA Dongwei. Iron-nitrogen-codoped Mesoporous Carbon: Facile Synthesis and Catalytic Performance of Oxygen Reduction Reaction[J]. Journal of Inorganic Materials, 2023, 38(11): 1309-1315.
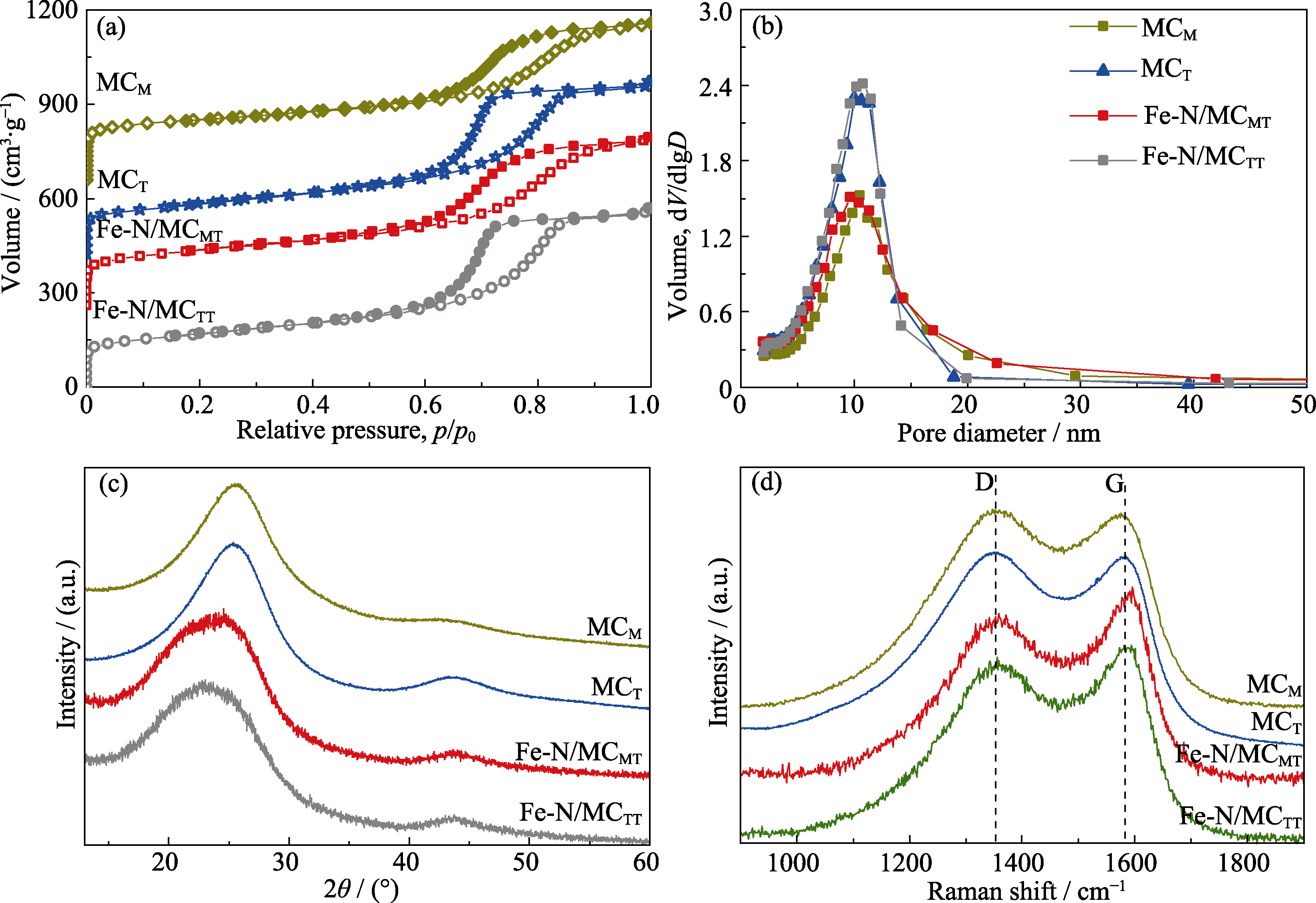
图2 MCM、MCT、Fe-N/MCMT和Fe-N/MCTT的(a)N2吸附-脱附等温线、(b)孔径分布图、(c)XRD图谱和(d)拉曼光谱图
Fig. 2 (a) N2 adsorption-desorption isotherms, (b) pore-size distributions, (c) XRD patterns, and (d) Raman spectra of MCM, MCT, Fe-N/MCMT and Fe-N/MCTT
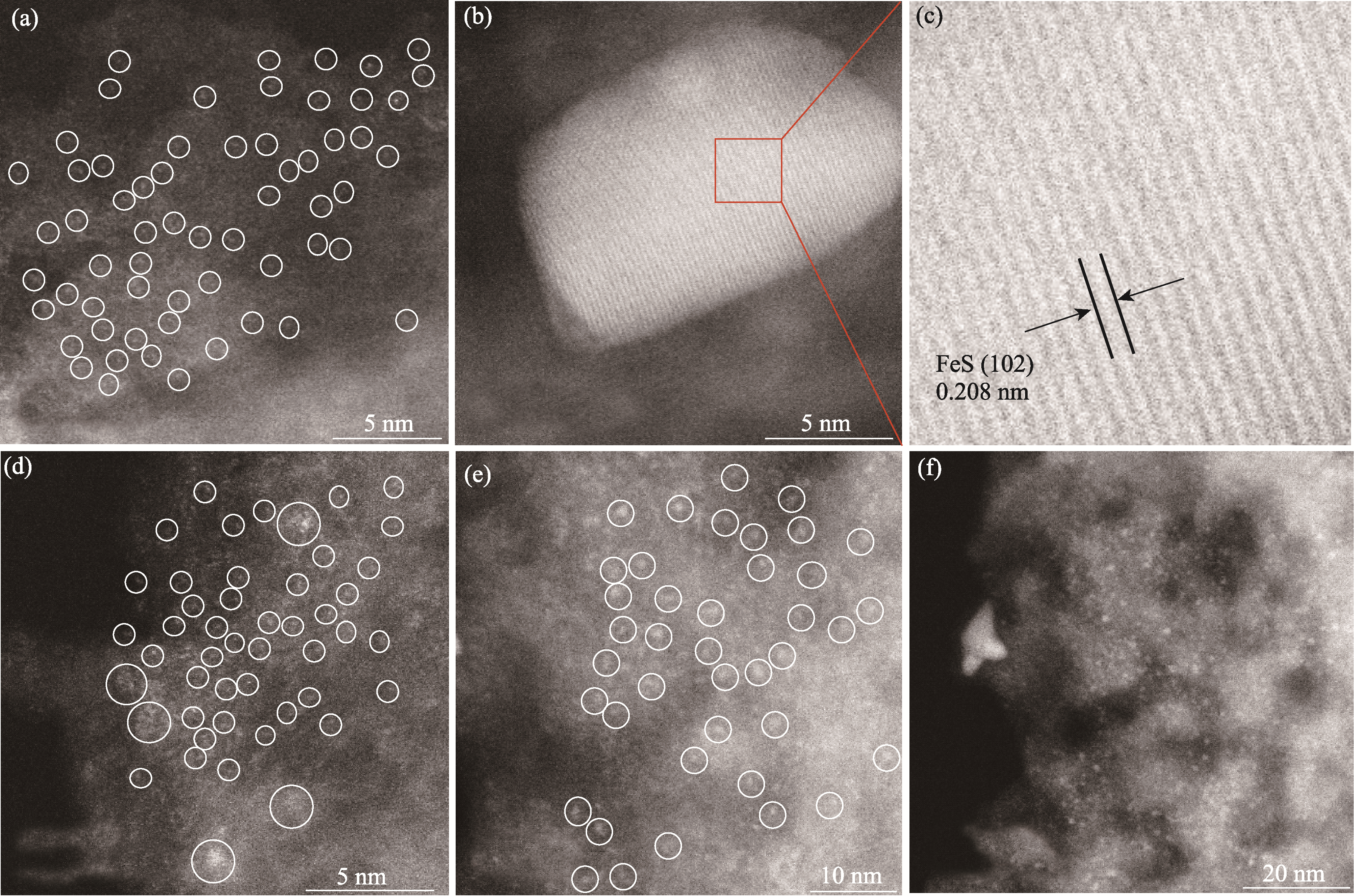
图3 (a~c)Fe-N/MCMT,(d~f)Fe-N/MCTT的HAADF-STEM照片
Fig. 3 HAADF-STEM images of (a-c) Fe-N/MCMT and (d-f) Fe-N/MCTT Single Fe atoms and Fe atom clusters are highlighted by white circles, respectively. Colorful figures are available on website
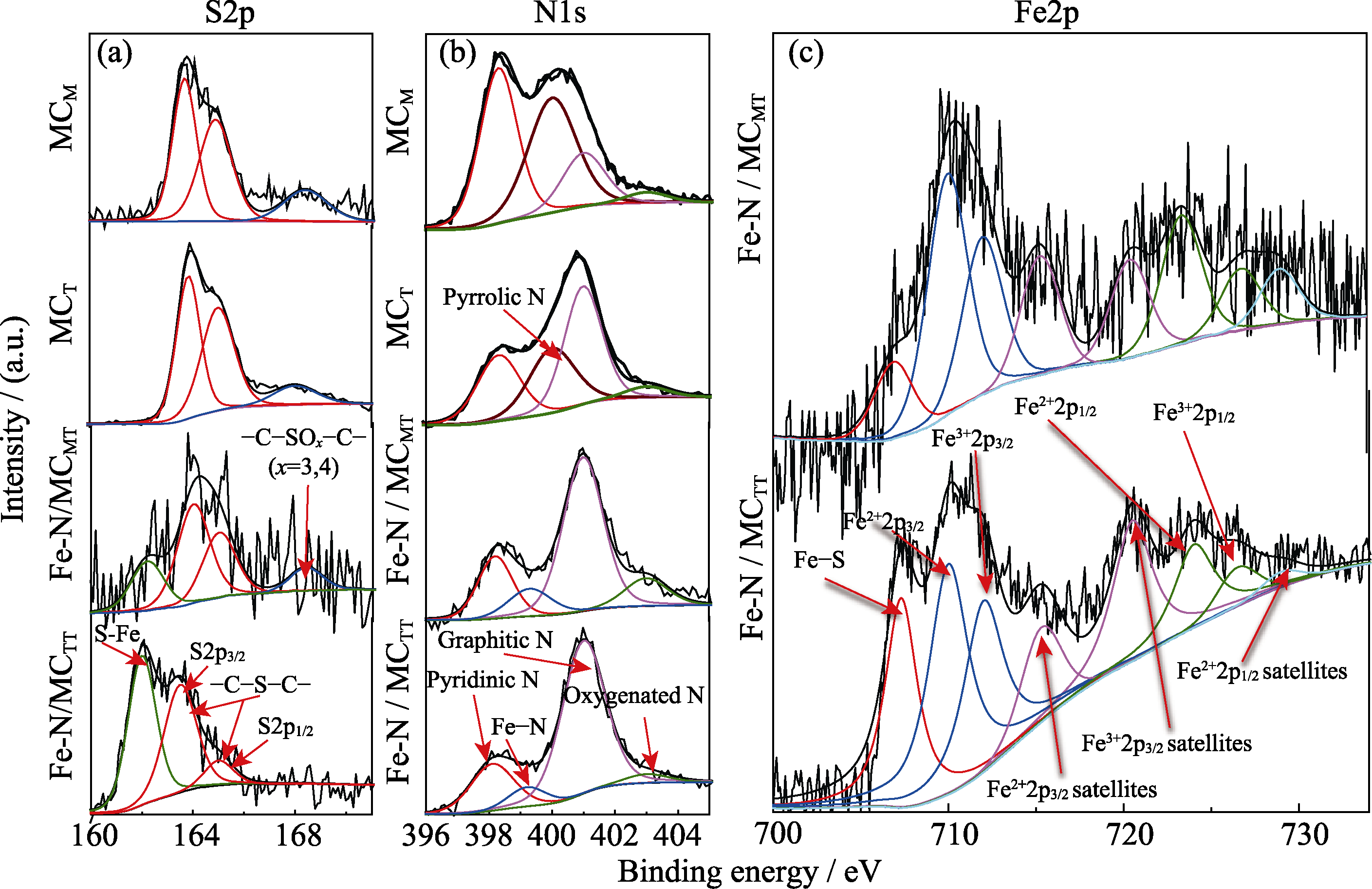
图4 MCM、MCT、Fe-N/MCMT和Fe-N/MCTT的高分辨率(a)N1s、(b)S2p和(c)Fe2p XPS光谱
Fig. 4 High-resolution (a) N1s, (b) S2p and (c) Fe2p XPS spectra of MCM, MCT, Fe-N/MCMT, and Fe-N/MCTT
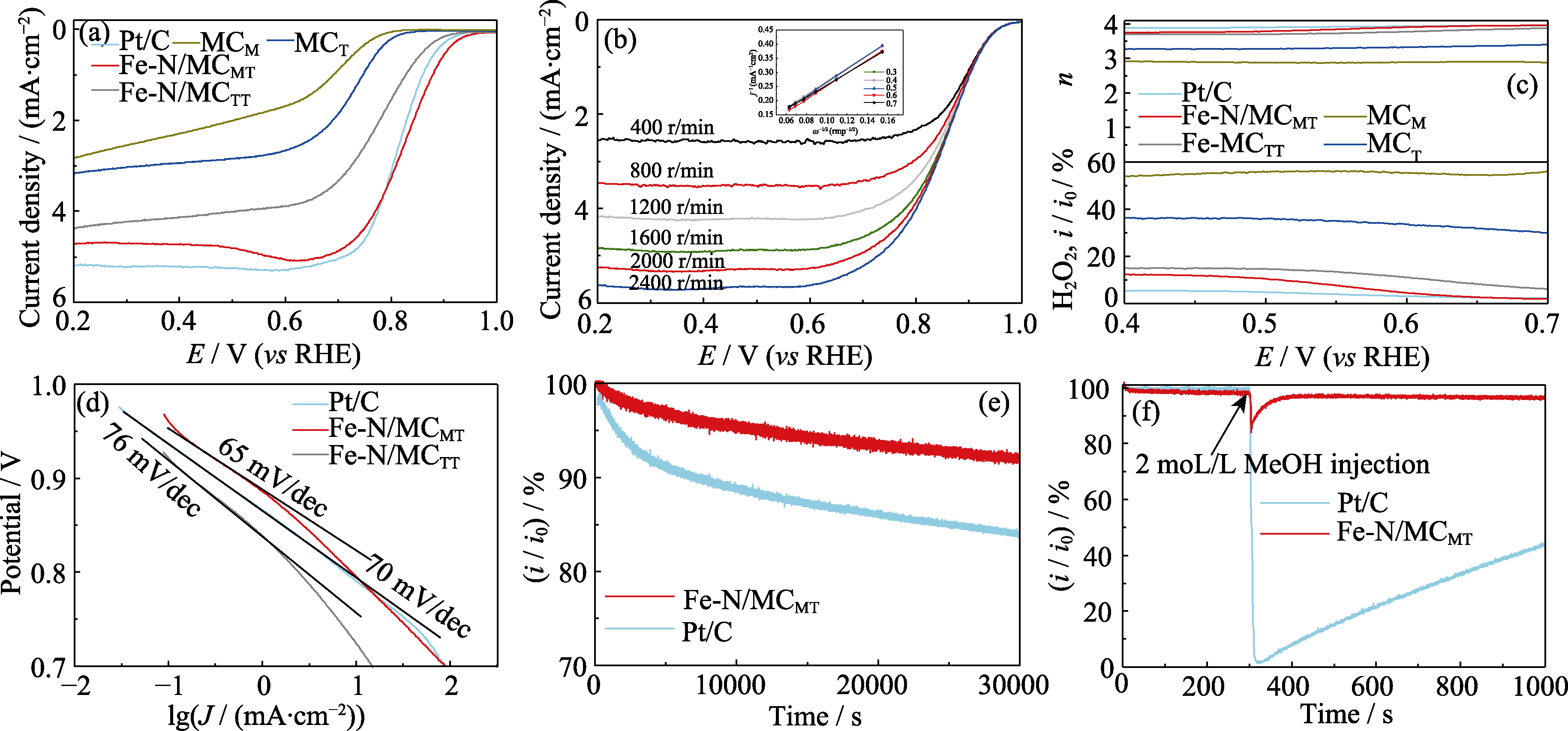
图5 各催化剂的ORR催化性能
Fig. 5 ORR performance of catalysts (a) LSV curves of different catalysts in O2-saturated 0.1 mol/L KOH at a scan rate of 10 mV/s and a rotation rate of 1600 r/min; (b) LSV curves of Fe-N/MCMT at different rotation rates with inset showing K-L plots obtained from polarization curves; (c) Plots of number of electron transfer and H2O2 yield with different catalysts at the rotation speed of 1600 r/min; (d) Tafel plots derived from Fig. 6(a); (e, f) Chronoamperometric responses of Fe-N/MCMT and Pt/C in (e) presence or (f) absence of methanol at 0.7 V (vs RHE). Colorful figures are available on website
| Samples | N/%(in atomic) | Binding energy of relative nitrogen content/eV | ||||
|---|---|---|---|---|---|---|
| Pyridinic N | Fe-Nx | Pyrollic N | Graphitic N | Oxygenated N | ||
| Fe-N/MCMT | 5.92 | 0.2 (398.2) | 0.11 ( 399.3) | 0.58 (401) | 0.11 (403) | |
| Fe-N/MCTT | 5.12 | 0.21 (398.1) | 0.07 ( 399.2) | 0.68 (401) | 0.04 (403) | |
| MCM | 16.48 | 0.41 (398.3) | 0.39 (400) | 0.16 (401) | 0.04 (403) | |
| MCT | 10.00 | 0.26 (398.3) | 0.28 (400) | 0.42 (400.98) | 0.04 (403) | |
表S1 各样品的氮含量
Table 1 Nitrogen content of each sample
| Samples | N/%(in atomic) | Binding energy of relative nitrogen content/eV | ||||
|---|---|---|---|---|---|---|
| Pyridinic N | Fe-Nx | Pyrollic N | Graphitic N | Oxygenated N | ||
| Fe-N/MCMT | 5.92 | 0.2 (398.2) | 0.11 ( 399.3) | 0.58 (401) | 0.11 (403) | |
| Fe-N/MCTT | 5.12 | 0.21 (398.1) | 0.07 ( 399.2) | 0.68 (401) | 0.04 (403) | |
| MCM | 16.48 | 0.41 (398.3) | 0.39 (400) | 0.16 (401) | 0.04 (403) | |
| MCT | 10.00 | 0.26 (398.3) | 0.28 (400) | 0.42 (400.98) | 0.04 (403) | |
| Sample | XPS | ||||
|---|---|---|---|---|---|
| Fe/% | N/% | S/% | O/% | C/% | |
| Fe-N/MCMT | 0.49 | 5.92 | 0.32 | 8.41 | 84.86 |
| Fe-N/MCTT | 0.64 | 5.12 | 0.57 | 9.34 | 84.33 |
| MCM | 0 | 16.48 | 1.27 | 7.92 | 74.33 |
| MCT | 0 | 10 | 0.9 | 5 | 84.1 |
表S2 各样品元素的原子百分比
Table 2 Elemental percentages in atom of each sample
| Sample | XPS | ||||
|---|---|---|---|---|---|
| Fe/% | N/% | S/% | O/% | C/% | |
| Fe-N/MCMT | 0.49 | 5.92 | 0.32 | 8.41 | 84.86 |
| Fe-N/MCTT | 0.64 | 5.12 | 0.57 | 9.34 | 84.33 |
| MCM | 0 | 16.48 | 1.27 | 7.92 | 74.33 |
| MCT | 0 | 10 | 0.9 | 5 | 84.1 |
| [1] |
JIANG L, XU S, XIA B, et al. Defect engineering of graphene hybrid catalysts for oxygen reduction reactions. J. Inorg. Mater., 2022, 37(2): 215.
DOI |
| [2] |
KIM D, ZUSSBLATT N P, CHUNG H T, et al. Highly graphitic mesoporous Fe, N-doped carbon materials for oxygen reduction electrochemical catalysts. ACS Appl. Mater. Interfaces, 2018, 10(30): 25337.
DOI URL |
| [3] |
SUN Y T, DING S, XU S S, et al. Metallic two-dimensional metal-organic framework arrays for ultrafast water splitting. J. Power Sources. 2021, 494: 229733.
DOI URL |
| [4] |
RAMASWAMY N, TYLUS U, JIA Q Y, et al. Activity descriptor identification for oxygen reduction on nonprecious electrocatalysts: linking surface science to coordination chemistry. J. Am. Chem. Soc., 2013, 135(41): 15443.
DOI PMID |
| [5] |
LEE S H, KIM J, CHUNG D Y, et al. Design principle of Fe-N-C electrocatalysts: how to optimize multimodal porous structures? J. Am. Chem. Soc., 2019, 141(5): 2035.
DOI PMID |
| [6] |
KONG A G, ZHU X F, HAN Z, et al. Ordered hierarchically micro- and mesoporous Fe-Nx-embedded graphitic architectures as efficient electrocatalysts for oxygen reduction reaction. ACS Catal., 2014, 4(6): 1793.
DOI URL |
| [7] |
NISHIHARA H, KYOTANI T. Templated nanocarbons for energy storage. Adv. Mater., 2012, 24(33): 4473.
DOI URL |
| [8] |
PENG Y, LU B Z, CHEN S W. Carbon-supported single atom catalysts for electrochemical energy conversion and storage. Adv. Mater., 2018, 30(48): 1801995.
DOI URL |
| [9] |
LEE J S, PARK G, KIM S T, et al. A highly efficient electrocatalyst for the oxygen reduction reaction: N-doped ketjenblack incorporated into Fe/Fe3C-functionalized melamine foam. Angew. Chem. Int. Ed., 2013, 52(3): 1026.
DOI URL |
| [10] |
YANG L, CHENG D J, ZENG X F, et al. Unveiling the high- activity origin of single-atom iron catalysts for oxygen reduction reaction. Proc. Natl. Acad. Sci. U.S.A., 2018, 115(26): 6626.
DOI URL |
| [11] |
LU X, YIM W L, SURYANTO B H R, et al. Electrocatalytic oxygen evolution at surface-oxidized multiwall carbon nanotubes. J. Am. Chem. Soc., 2015, 137(8): 2901.
DOI PMID |
| [12] |
LIU J, JIAO M G, MEI B B, et al. Carbon-supported divacancy- anchored platinum single-atom electrocatalysts with superhigh Pt utilization for the oxygen reduction reaction. Angew. Chem. Int. Ed., 2019, 131(4): 1175.
DOI URL |
| [13] |
ZHANG S G, MANDAI T, UENO K, et al. Hydrogen-bonding spramolecular protic salt as an “all-in-one” precursor for nitrogen- doped mesoporous carbons for CO2 adsorption. Nano Energy, 2015, 13: 376.
DOI URL |
| [14] |
XING C, YANG D H, ZHANG Y T, et al. Semi-closed synthesis of nitrogen and oxygen co-doped mesoporous carbon for selective aqueous oxidation. Green Energy Environ., 2022, 7(1): 43.
DOI URL |
| [15] |
SING K S. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl. Chem., 1985, 57(4): 603.
DOI URL |
| [16] | DAS A, CHAKRABORTY B, SOOD A K B. Raman spectroscopy of graphene on different substrates and influence of defects. Mater. Sci., 2008, 31: 579. |
| [17] |
WU Z Y, XU X X, HU B C, et al. Iron carbide nanoparticles encapsulated in mesoporous Fe-N-doped carbon nanofibers for efficient electrocatalysis. Angew. Chem. Int. Ed., 2015, 54(28): 8179.
DOI URL |
| [18] |
SUN M, DAVENPORT D, LIU H J, et al. Highly efficient and sustainable non-precious-metal Fe-N-C electrocatalysts for the oxygen reduction reaction. J. Mater. Chem. A, 2018, 6(6): 2527.
DOI URL |
| [19] |
SEROV A, ARTYUSHKOVA K, ATANASSOV P. Fe-N-C oxygen reduction fuel cell catalyst derived from carbendazim: synthesis, structure, and reactivity. Adv. Energy Mater., 2014, 4(10): 1301735.
DOI URL |
| [20] |
ZHAO Y X, LAI Q X, WANG Y, et al. Interconnected hierarchically porous Fe, N-codoped carbon nanofibers as efficient oxygen reduction catalysts for Zn-air batteries. ACS Appl. Mater. Interfaces, 2017, 9(19): 16178.
DOI URL |
| [21] |
DING Y J, NIU Y C, YANG J, et al. A metal-amino acid complex- derived bifunctional oxygen electrocatalyst for rechargeable zinc-air batteries. Small, 2016, 12(39): 5414.
DOI URL |
| [22] |
LEFEVRE M, PROIETTI E, JAOUEN F, et al. Iron-based catalysts with improved oxygen reduction activity in polymer electrolyte fuel cells. Science, 2009, 324(5923): 71.
DOI PMID |
| [23] |
LI J, SONG Y J, ZHANG G X, et al. Multicolor printing using electric-field-responsive and photocurable photonic crystals. Adv. Funct. Mater., 2017, 27(43): 1702825.
DOI URL |
| [24] |
CAO L, LI Z H, GU Y, et al. Rational design of n-doped carbon nanobox-supported Fe/Fe2N/Fe3C nanoparticles as efficient oxygen reduction catalysts for Zn-air batteries. J. Mater. Chem. A, 2017, 5(22): 11340.
DOI URL |
| [1] | 王磊, 李建军, 宁军, 胡天玉, 王洪阳, 张占群, 武琳馨. CoFe2O4@Zeolite催化剂活化过一硫酸盐对甲基橙的强化降解: 性能与机理[J]. 无机材料学报, 2023, 38(4): 469-476. |
| [2] | 王如意, 徐国良, 杨蕾, 邓崇海, 储德林, 张苗, 孙兆奇. p-n异质结BiVO4/g-C3N4光阳极的制备及其光电化学水解性能[J]. 无机材料学报, 2023, 38(1): 87-96. |
| [3] | 姚仪帅, 郭瑞华, 安胜利, 张捷宇, 周国治, 张国芳, 黄雅荣, 潘高飞. 原位负载Pt-Co高指数晶面催化剂的制备及其电催化性能[J]. 无机材料学报, 2023, 38(1): 71-78. |
| [4] | 陈瀚翔, 周敏, 莫曌, 宜坚坚, 李华明, 许晖. CoN/g-C3N4 0D/2D复合结构及其光催化制氢性能研究[J]. 无机材料学报, 2022, 37(9): 1001-1008. |
| [5] | 胡越, 安琳, 韩鑫, 侯成义, 王宏志, 李耀刚, 张青红. RhO2修饰BiVO4薄膜光阳极的制备及其光电催化分解水性能[J]. 无机材料学报, 2022, 37(8): 873-882. |
| [6] | 孙炼, 顾全超, 杨雅萍, 王洪磊, 余金山, 周新贵. 二维过渡金属硫属化合物氧还原反应催化剂的研究进展[J]. 无机材料学报, 2022, 37(7): 697-709. |
| [7] | 安琳, 吴淏, 韩鑫, 李耀刚, 王宏志, 张青红. 非贵金属Co5.47N/N-rGO助催化剂增强TiO2光催化制氢性能[J]. 无机材料学报, 2022, 37(5): 534-540. |
| [8] | 王虹力, 王男, 王丽莹, 宋二红, 赵占奎. 功能化石墨烯担载型AuPd纳米催化剂增强甲酸制氢反应[J]. 无机材料学报, 2022, 37(5): 547-553. |
| [9] | 蒋丽丽, 徐帅帅, 夏宝凯, 陈胜, 朱俊武. 缺陷调控石墨烯复合催化剂在氧还原反应中的作用[J]. 无机材料学报, 2022, 37(2): 215-222. |
| [10] | 高娃, 熊宇杰, 吴聪萍, 周勇, 邹志刚. 基于超薄纳米结构的光催化二氧化碳选择性转化[J]. 无机材料学报, 2022, 37(1): 3-14. |
| [11] | 王潇, 朱智杰, 吴之怡, 张城城, 陈志杰, 肖梦琦, 李超然, 何乐. 钴等离激元超结构粉体催化剂的制备及其光热催化应用[J]. 无机材料学报, 2022, 37(1): 22-28. |
| [12] | 郭李娜, 何雪冰, 吕琳, 吴丹, 原弘. 调控CuO表面性质选择性电催化还原CO2制HCOOH[J]. 无机材料学报, 2022, 37(1): 29-37. |
| [13] | 刘自若, 刘炜, 郝策, 胡金文, 史彦涛. 蜂窝状碳负载铁基单原子催化剂的制备及ORR催化性能研究[J]. 无机材料学报, 2021, 36(9): 943-949. |
| [14] | 郝策, 刘自若, 刘炜, 史彦涛. 用于氧还原反应的碳基负载金属单原子催化剂研究进展[J]. 无机材料学报, 2021, 36(8): 820-834. |
| [15] | 范君, 江雪, 焦毅, 陈宇圣, 王健礼, 陈耀强. 不同碱辅助的沉积沉淀法对三效催化剂稳定性的影响[J]. 无机材料学报, 2021, 36(6): 659-664. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||