无机材料学报 ›› 2022, Vol. 37 ›› Issue (11): 1151-1169.DOI: 10.15541/jim20220194
所属专题: 【生物材料】肿瘤治疗
收稿日期:2022-04-04
修回日期:2022-05-02
出版日期:2022-11-20
网络出版日期:2022-06-16
通讯作者:
陈雨, 教授. E-mail: chenyuedu@shu.edu.cn作者简介:黄慧(1994-), 女, 博士研究生. E-mail: huanghuiscu@sina.com
基金资助:
HUANG Hui1,2( ), CHEN Yu1,2,3(
), CHEN Yu1,2,3( )
)
Received:2022-04-04
Revised:2022-05-02
Published:2022-11-20
Online:2022-06-16
Contact:
CHEN Yu, professor. E-mail: chenyuedu@shu.edu.cnAbout author:HUANG Hui (1994-), female, PhD candidate. E-mail: huanghuiscu@sina.com
Supported by:摘要:
临床医学和生物材料的蓬勃发展, 促进了多种疾病的诊断成像、有效治疗和精准诊疗。材料与医学交叉学科(简称“材料医学”)的发展旨在克服传统临床医学面临的主要障碍和挑战, 如系统性毒性、生物利用度差、靶向部位特异性低、诊断/治疗效果不理想等。本文系统地阐述了近年来各种医学材料在疾病诊断、治疗和诊疗方面的应用进展, 特别是纳米医学材料的研究进展。首先, 重点讨论癌症治疗领域的生物医学成像(如光学成像、磁共振成像、超声成像、计算机断层成像等)和治疗策略(如光热治疗、动力学治疗、免疫治疗、协同治疗等)。此外, 我们还重点介绍了医学材料对骨组织工程、呼吸系统、中枢神经系统等疾病的诊断和治疗的最新进展, 并重点阐述了用于生物传感和抗微生物等其他代表性生物医学领域的医学材料。最后, 我们讨论了这些独特的医学材料在实际临床转化和应用中所面临的挑战和未来的机遇, 以促进其早日实现临床转化, 推动医学进步和造福患者。
中图分类号:
黄慧, 陈雨. 材料医学和医学材料[J]. 无机材料学报, 2022, 37(11): 1151-1169.
HUANG Hui, CHEN Yu. Materdicine and Medmaterial[J]. Journal of Inorganic Materials, 2022, 37(11): 1151-1169.
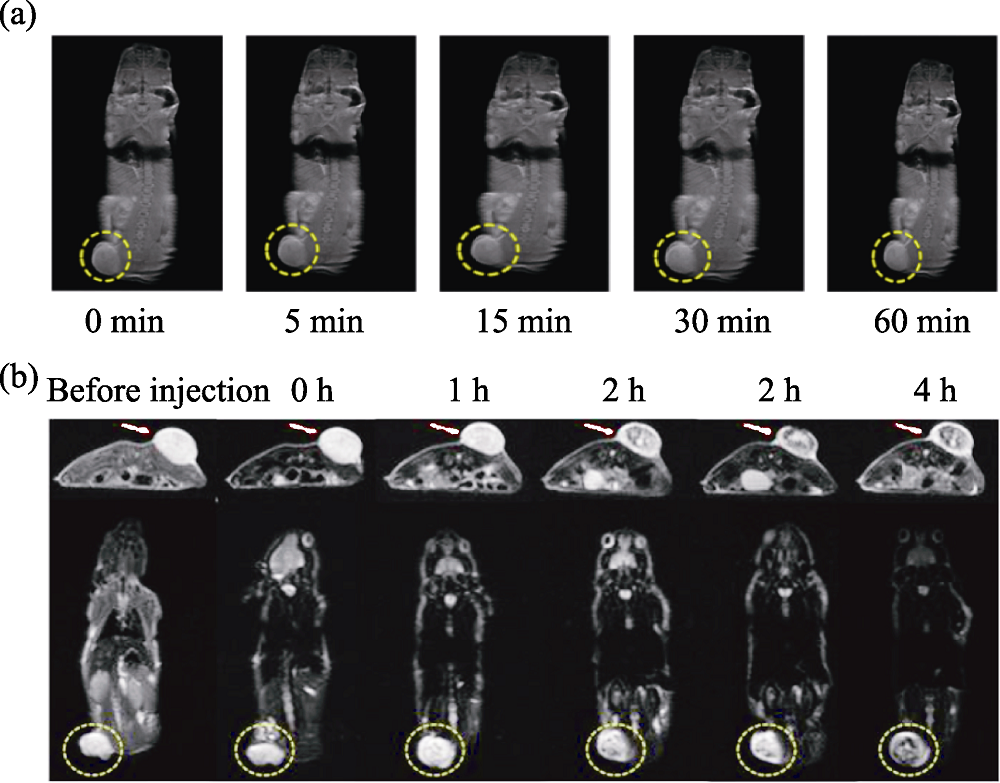
图1 医学材料用于磁共振成像[33,35]
Fig. 1 Medmaterials for magnetic resonance imaging [33,35] (a) T1-weighted magnetic resonance imaging of tumor-bearing mice after intravenous injection of Mn-based nanomaterials; (b) T2-weighted magnetic resonance imaging of tumor-bearing mice after intravenous injection of iron oxide-based nanomaterials
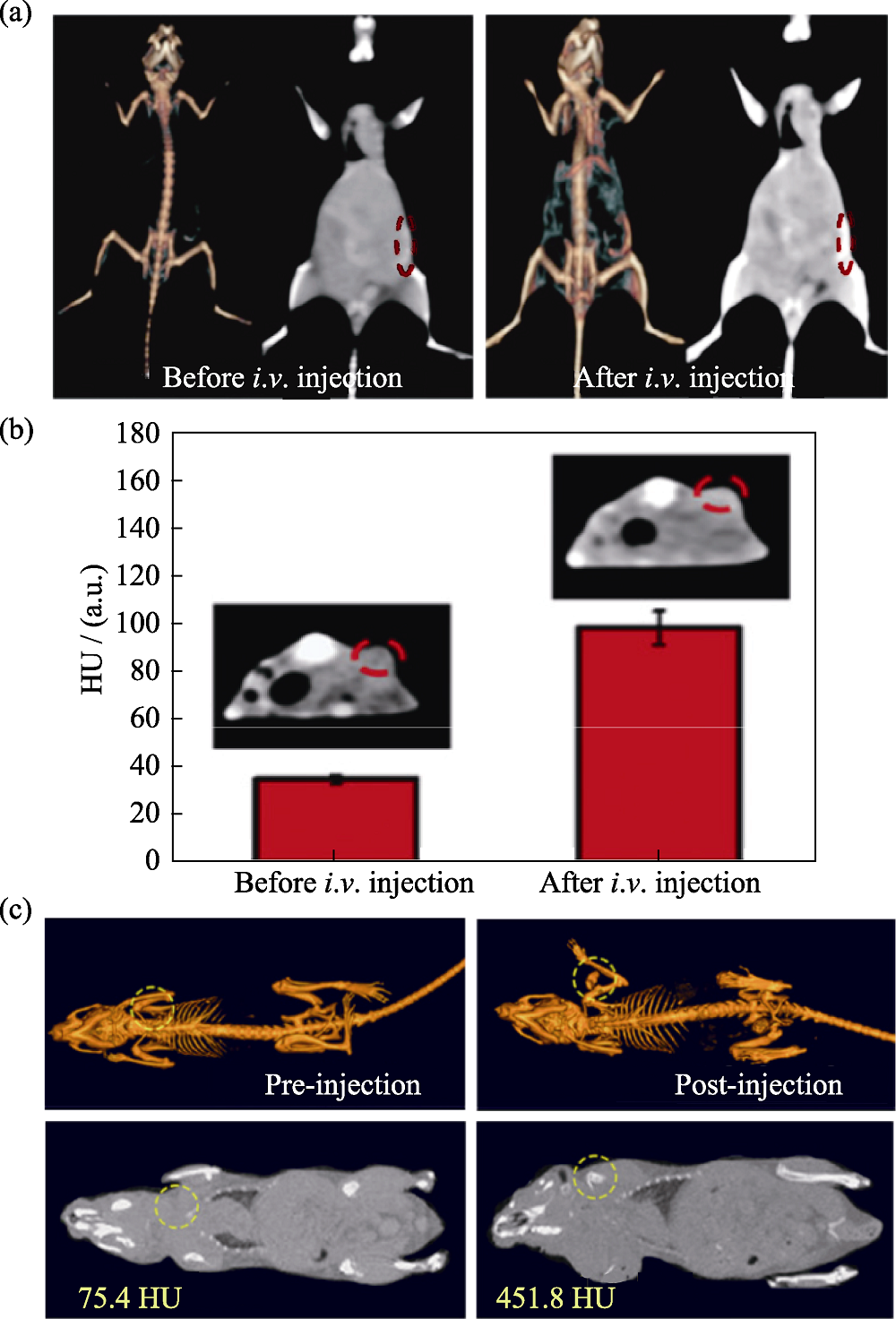
图2 医学材料用于计算机断层扫描成像[43-44]
Fig. 2 Medmaterials for computed tomography imaging [43-44] (a) Computed tomography imaging in vivo before and after intravenous administration of W1.33C nanosheets; (b) Signal intensities of computed tomography imaging and corresponding coronal plane images (inset) before and after intravenous administration; (c) In vivo computed tomography images of tumor-bearing mice before and after injection of copper/manganese silicate nanosphere-coated lanthanide-doped nanoparticles. HU: Hounsfield unit
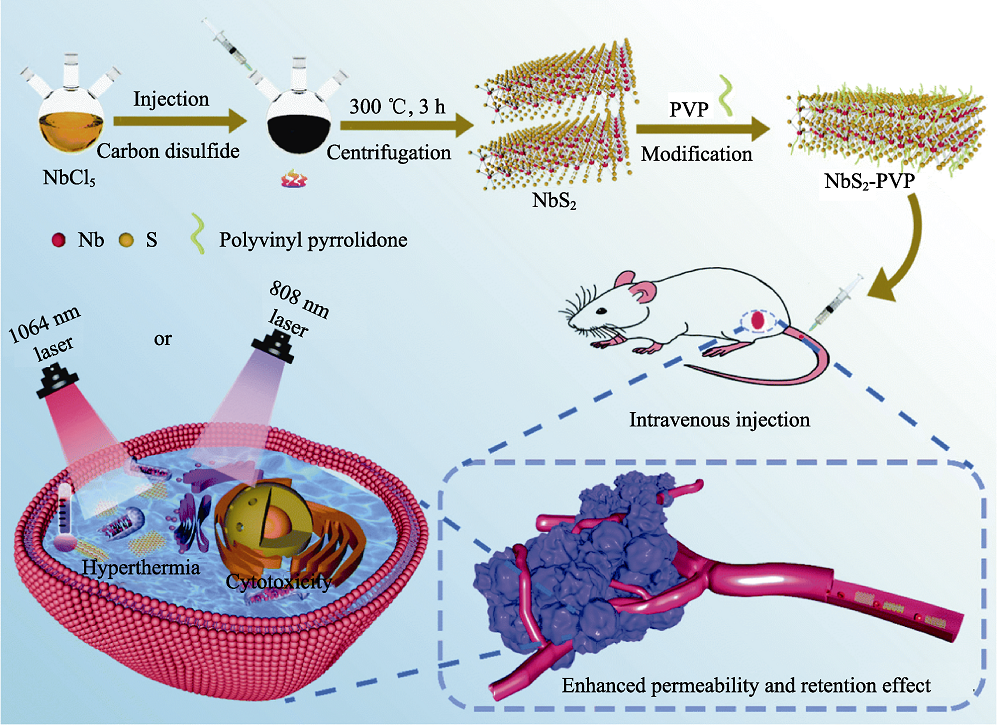
图3 医学材料用于肿瘤光热治疗[55]
Fig. 3 Medmaterials for photothermal therapy against cancer[55] Scheme illustrating the NbS2 nanosheets-based photothermal tumor therapy in the first near-infrared and second near-infrared biowindows. PVP: Polyvinyl pyrrolidone
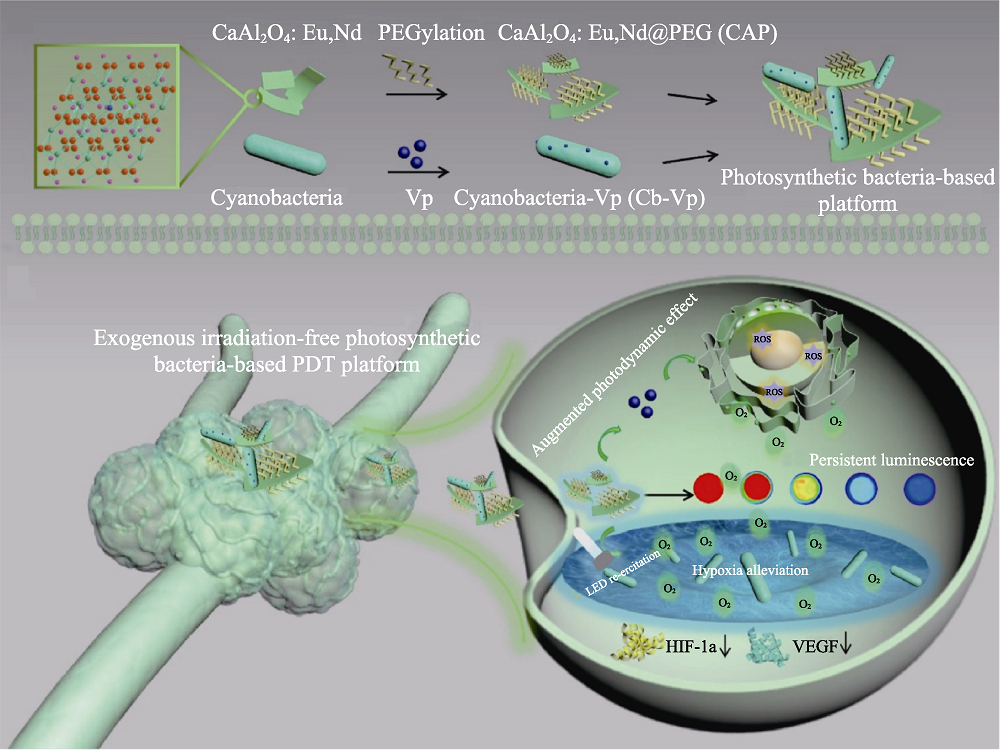
图4 医学材料用于肿瘤光动力学治疗[61]
Fig. 4 Medmaterials for photodynamic therapy against cancer[61] Schematic representation of the exogenous irradiation-free photosynthetic bacteria-based system for photodynamic tumor therapy. PDT: Photodynamic therapy; HIF: Hypoxia inducible factor; VEGF: Vascular endothelial growth factor
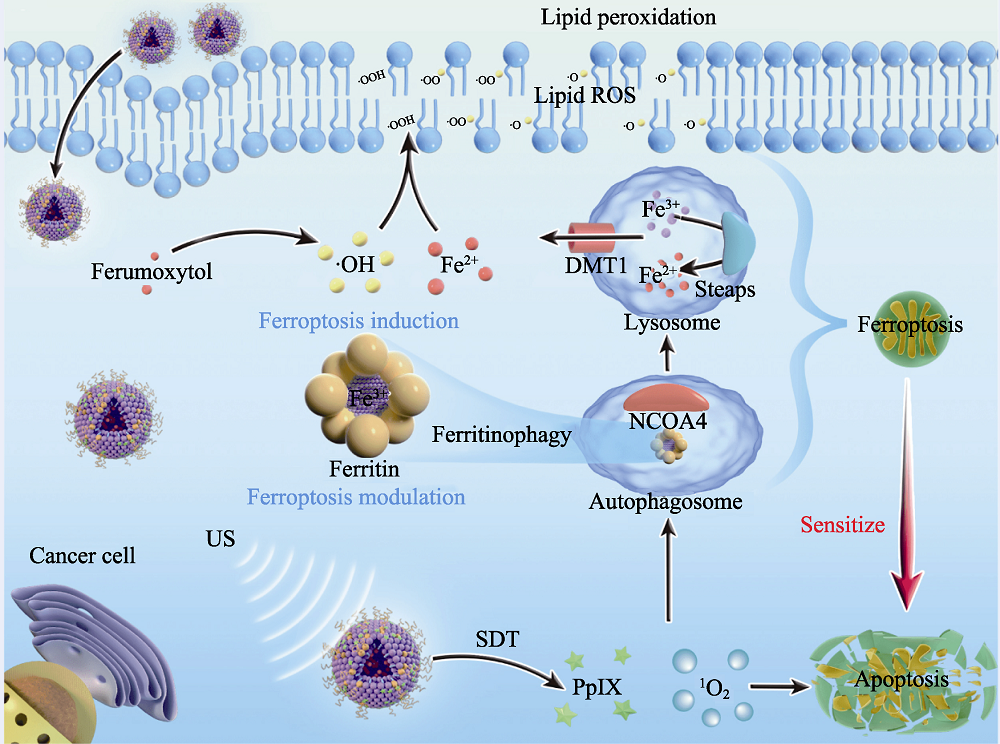
图5 医学材料用于肿瘤声动力治疗[62]
Fig. 5 Medmaterials for tumor sonodynamic therapy [62] Scheme of the underlying therapeutic mechanism of sonodynamic therapy-based ferroptosis-targeting. NCOA4: Nuclear receptor coactivator 4; US: Ultrasound; SDT: Sonodynamic therapy; PpIX: Protoporphyrin IX; DMT1: Divalent metal transporter 1; STEAPS: Six-transmembrane epithelial antigen of the prostate
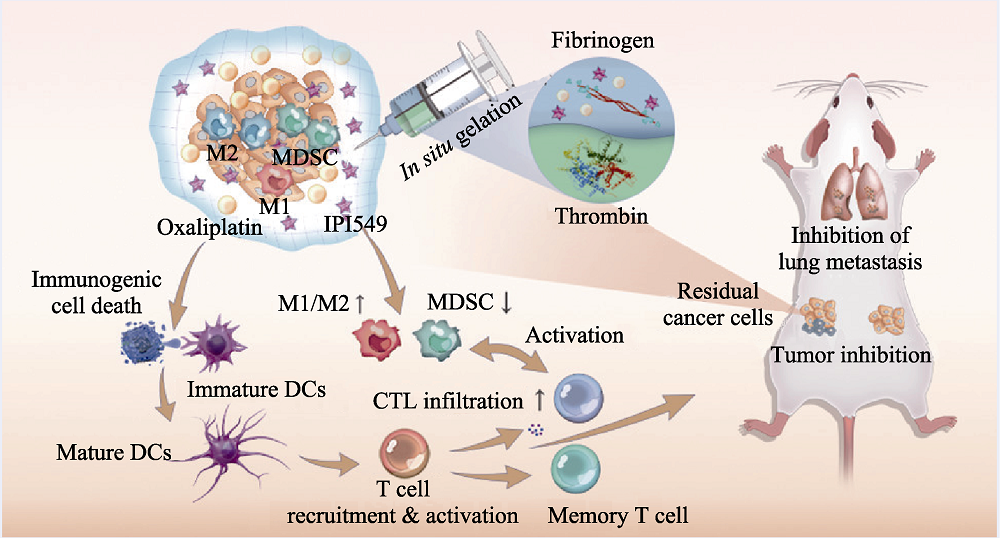
图6 医学材料用于肿瘤免疫治疗[63]
Fig. 6 Medmaterials for tumor immunotherapy[63] Scheme demonstrating the mechanism of chemoimmunotherapeutic approach for inhibiting tumor growth and metastasis M1: M1-like macrophages; M2: M2-like macrophages; MDSC: Myeloid-derived suppressor cells; IPI549: Selective PI3Kγ inhibitor verified in multiple tumor models; DC: Dendritic cells; CTL: Cytotoxic T lymphocytes
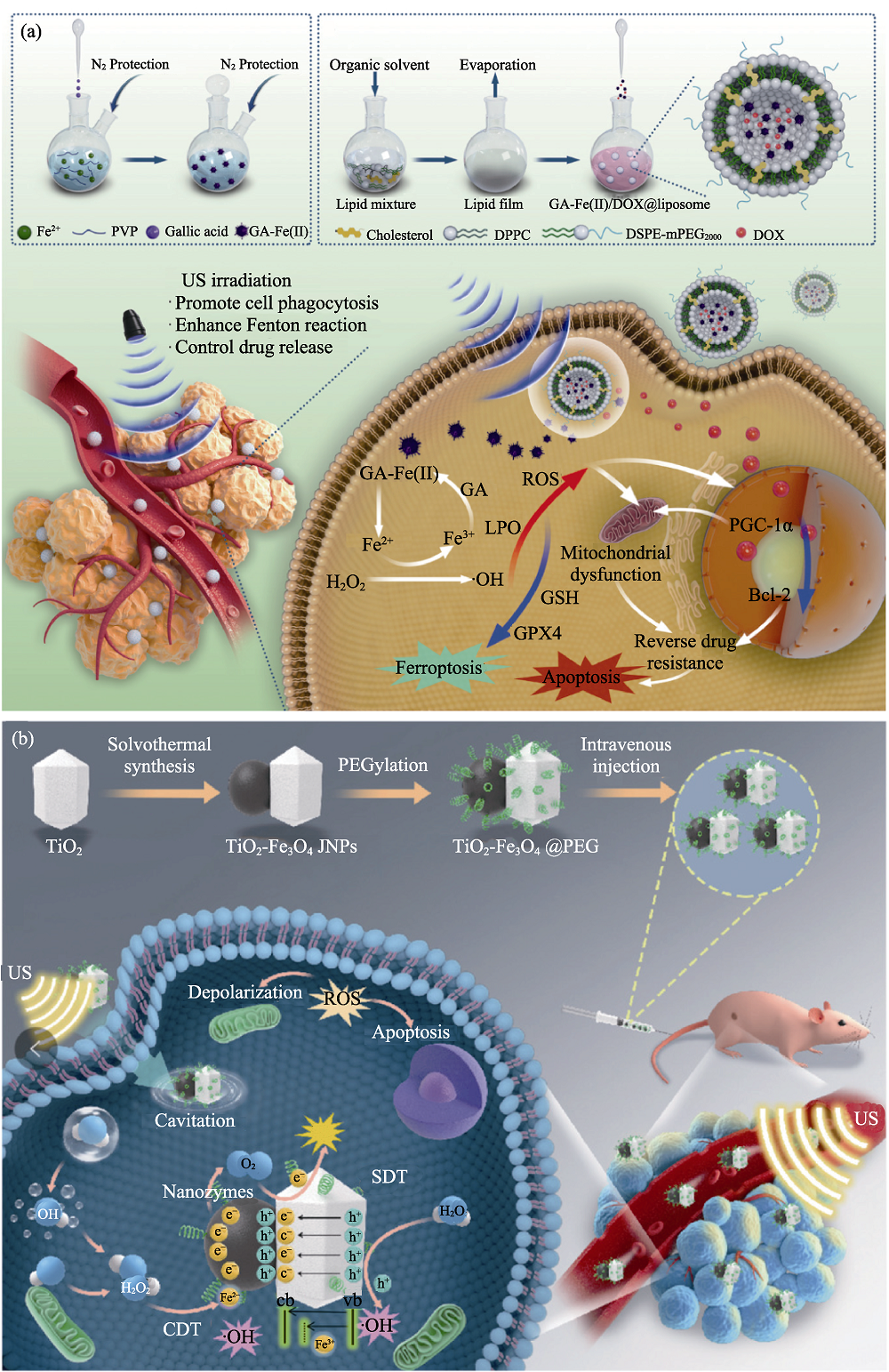
图7 医学材料用于肿瘤协同治疗[74-75]
Fig. 7 Medmaterials for tumor synergistic therapy[74-75] (a) Scheme illustrating the function of GA-Fe(II)/DOX@liposome for reversing drug resistance by ultrasound-augmented nanocatalytic ferroptosis; (b) Schematic illustration of the synergistic enhancement of chemodynamic and sonodynamic therapy mediated by TiO2-Fe3O4 Janus nanosonosensitizers. LPO: Lipid peroxidation; GA: Gallic acid; US: Ultrasound; SDT: Sonodynamic therapy; CDT: Chemodynamic therapy
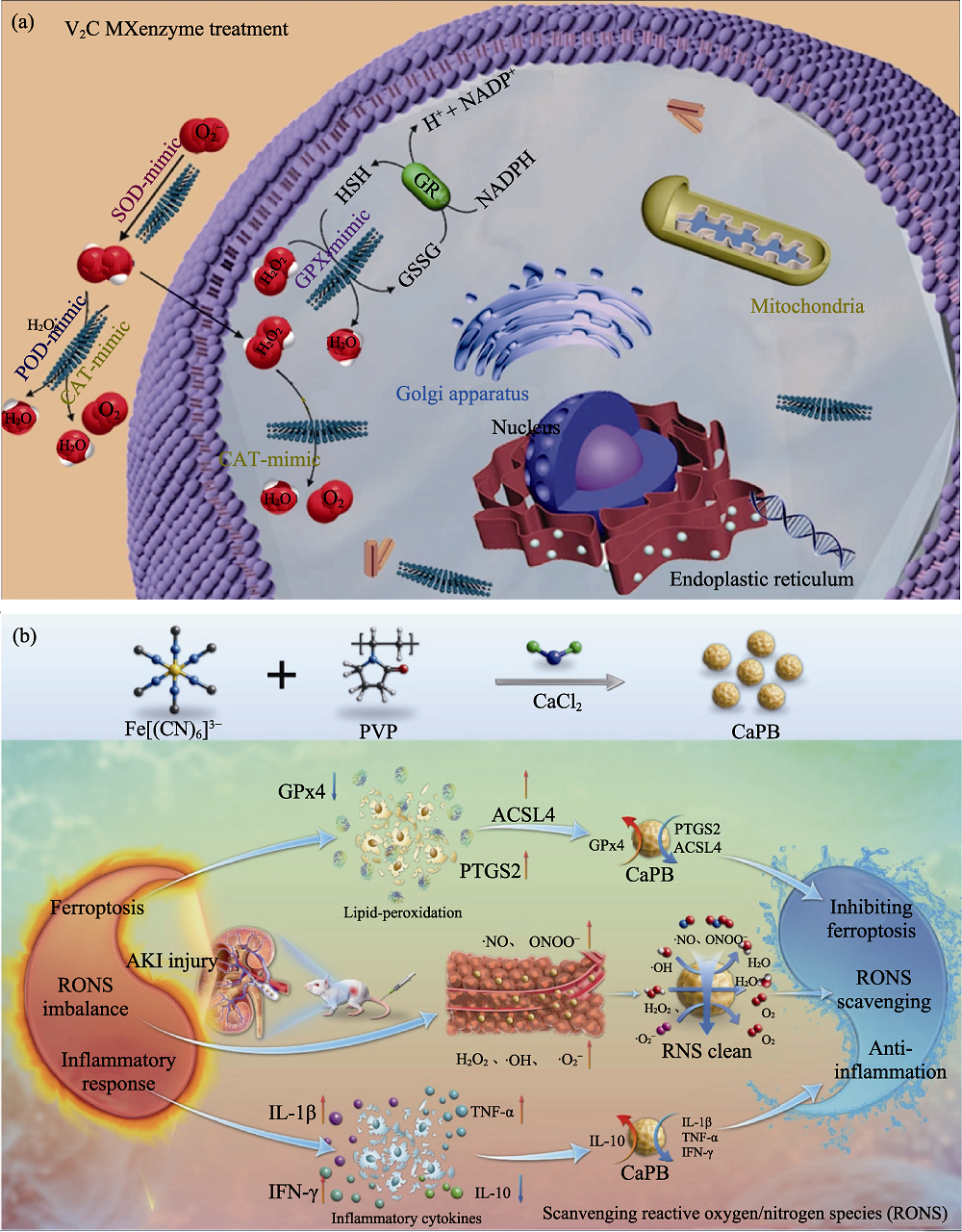
图8 医学材料用于活性氧清除相关疾病和急性肾损伤的诊疗[101-102]
Fig. 8 Medmaterials for theranostics of ROS-scavenging related diseases and acute kidney injury [101-102] (a) Scheme indicating ROS-scavenging activities of V2C MXene with multiple enzyme-like natures; (b) Scheme representing CaPB nanozymes in the acute kidney injury treatment. ROS: Reactive oxygen species; MXene: Transition metal carbides, carbonitrides and nitrides; GPx4: Glutathione peroxidase 4; ACSL4: Acyl-CoA synthetase long chain family member 4; PTGS2: Prostaglandin-endoperoxide synthase 2; RONS: Reactive oxygen and nitrogen species
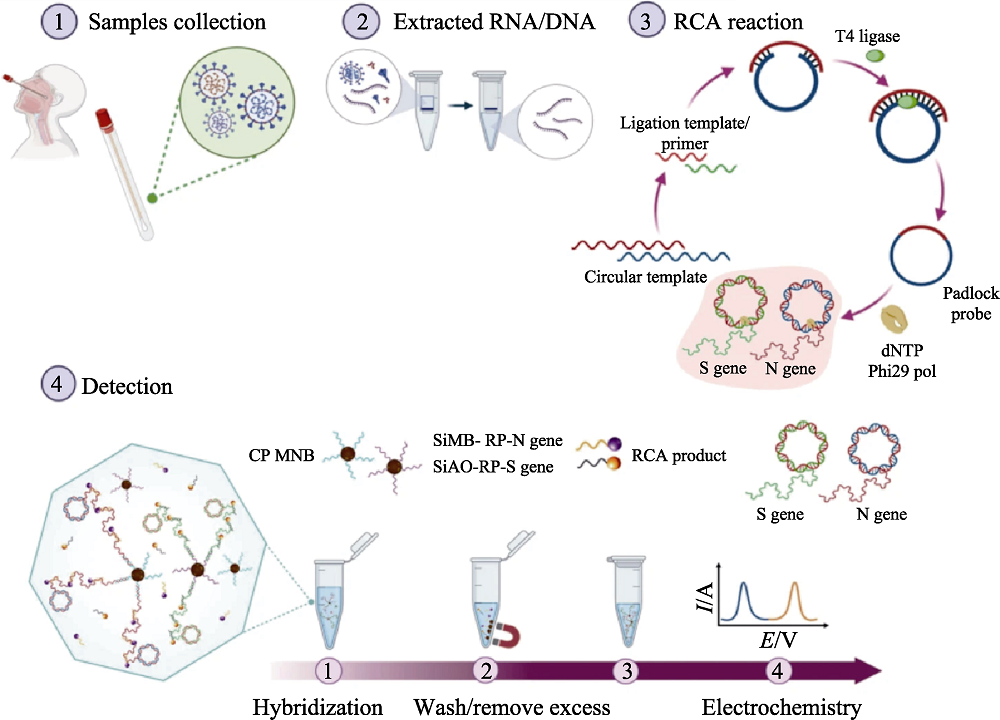
图9 医学材料用于生物传感[111]
Fig. 9 Medmaterials for biosensing[111] Scheme illustrating detection workflow of SARS-CoV-2 using the electrochemical biosensor RCA: Rolling circle amplification; CP MNB: Capture probe-conjugated magnetic bead particle; SiMB: Silica with a redox-dye layer; RP: Reporter probe
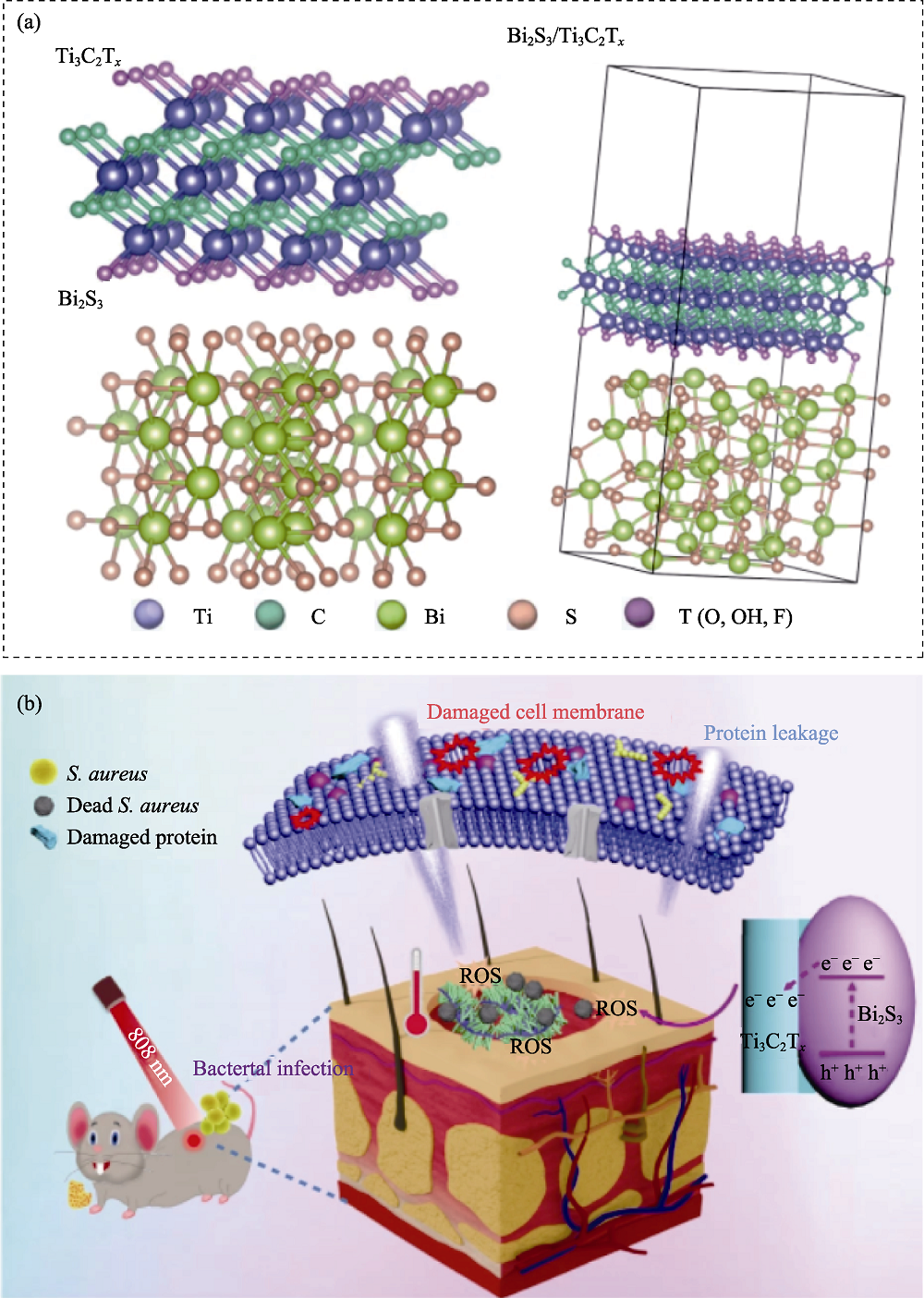
图10 医学材料用于抗菌[118]
Fig. 10 Medmaterials for antibacterial applications[118] (a) Crystal structure of Ti3C2/Bi2S3; (b) Schematic illustration of the antibacterial mechanism of Ti3C2/Bi2S3 under 808 nm laser irradiation
| [1] |
MALEKJAHANI A, SINDHWANI S, SYED A M, et al. Engineering steps for mobile point-of-care diagnostic devices. Accounts of Chemical Research, 2019, 52(9): 2406-2414.
DOI PMID |
| [2] |
HE H, LIU L, MORIN E E, et al. Survey of clinical translation of cancer nanomedicines—lessons learned from successes and failures. Accounts of Chemical Research, 2019, 52(9): 2445-2461.
DOI URL |
| [3] | HUANG H, FENG W, CHEN Y, et al. Inorganic nanoparticles in clinical trials and translations. Nano Today, 2020, 35: 100972-24. |
| [4] |
HUANG H, FENG W, CHEN Y. Two-dimensional biomaterials: material science, biological effect and biomedical engineering applications. Chemical Society Reviews, 2021, 50(20): 11381-11485.
DOI PMID |
| [5] |
PELAZ B, ALEXIOU C H, ALVAREZ-PUEBLA R A, et al. Diverse applications of nanomedicine. ACS Nano, 2017, 11(3): 2313-2381.
DOI PMID |
| [6] |
BURDA C, CHEN X, NARAYANAN R, et al. Chemistry and properties of nanocrystals of different shapes. Chemical Reviews, 2005, 105(4): 1025-1102.
PMID |
| [7] |
XIANG H, CHEN Y. Materdicine: interdiscipline of materials and medicine. VIEW, 2020, 1(3): 20200016-29.
DOI URL |
| [8] |
SHI J J, KANTOFF P W, WOOSTER R, et al. Cancer nanomedicine: progress, challenges and opportunities. Nature Reviews Cancer, 2017, 17(1): 20-37.
DOI PMID |
| [9] |
IRBY D, DU C, LI F. Lipid-drug conjugate for enhancing drug delivery. Molecular Pharmaceutics, 2017, 14(5): 1325-1338.
DOI PMID |
| [10] |
MURA S, NICOLAS J, COUVREUR P. Stimuli-responsive nanocarriers for drug delivery. Nature Materials, 2013, 12(11): 991-1003.
DOI PMID |
| [11] |
NICOLAS J, MURA S, BRAMBILLA D, et al. Design, functionalization strategies and biomedical applications of targeted biodegradable/biocompatible polymer-based nanocarriers for drug delivery. Chemical Society Reviews, 2013, 42(3): 1147-1235.
DOI PMID |
| [12] |
KUNJACHAN S, EHLING J, STORM G, et al. Noninvasive imaging of nanomedicines and nanotheranostics: principles, progress, and prospects. Chemical Reviews, 2015, 115(19): 10907-10937.
DOI PMID |
| [13] |
CHEN H M, ZHANG W Z, ZHU G Z, et al. Rethinking cancer nanotheranostics. Nature Reviews Materials, 2017, 2(7): 17024-18.
DOI URL |
| [14] |
WANG C, HUANG W, ZHOU Y, et al. 3D printing of bone tissue engineering scaffolds. Bioactive Materials, 2020, 5(1): 82-91.
DOI PMID |
| [15] | KAUR B, KUMAR S, KAUSHIK B K. Recent advancements in optical biosensors for cancer detection. Biosensors and Bioelectronics, 2022, 197: 113805-11. |
| [16] |
HUH A J, KWON Y J. “Nanoantibiotics”: a new paradigm for treating infectious diseases using nanomaterials in the antibiotics resistant era. Journal of Controlled Release, 2011, 156(2): 128-145.
DOI URL |
| [17] |
DUAN Y, LIU B. Recent advances of optical imaging in the second near-infrared window. Advanced Materials, 2018, 30(47): 1802394-19.
DOI URL |
| [18] |
HUANG L Y, ZHU S, CUI R, et al. Noninvasive in vivo imaging in the second near-infrared window by inorganic nanoparticle-based fluorescent probes. Analytical Chemistry, 2019, 92(1): 535-542.
DOI URL |
| [19] |
LIN H, GAO S, DAI C, et al. A two-dimensional biodegradable niobium carbide (MXene) for photothermal tumor eradication in NIR-I and NIR-II biowindows. Journal of the American Chemical Society, 2017, 139(45): 16235-16247.
DOI PMID |
| [20] |
YANG Q, HU Z, ZHU S, et al. Donor engineering for NIR-II molecular fluorophores with enhanced fluorescent performance. Journal of the American Chemical Society, 2018, 140(5): 1715-1724.
DOI PMID |
| [21] |
YANG H C, LI R F, ZHANG Y J, et al. Colloidal alloyed quantum dots with enhanced photoluminescence quantum yield in the NIR-II window. Journal of the American Chemical Society, 2021, 143(6): 2601-2607.
DOI PMID |
| [22] | FAN Y, WANG S, ZHANG F. Optical multiplexed bioassays for improved biomedical diagnostics. Angewandte Chemie International Edition, 2019, 131(38): 13342-13353. |
| [23] |
WELSHER K, LIU Z, SHERLOCK SP, et al. A route to brightly fluorescent carbon nanotubes for near-infrared imaging in mice. Nature Nanotechnology, 2009, 4(11): 773-780.
DOI PMID |
| [24] |
CHANG B, LI D, REN Y, et al. A phosphorescent probe for in vivo imaging in the second near-infrared window. Nature Biomedical Engineering, 2022, 6(6): 629-639.
DOI URL |
| [25] |
WANG Q, LIANG Z, LI F, et al. Dynamically switchable magnetic resonance imaging contrast agents. Exploration, 2021, 1(2): 20210009-8.
DOI URL |
| [26] |
KIM D, KIM J, PARK Y I, et al. Recent development of inorganic nanoparticles for biomedical imaging. ACS Central Science, 2018, 4(3): 324-336.
DOI PMID |
| [27] |
XU Z, LIU C, ZHAO S, et al. Molecular sensors for NMR-based detection. Chemical Reviews, 2018, 119(1): 195-230.
DOI URL |
| [28] |
CHO M H, SHIN S H, PARK S H, et al. Targeted, stimuli-responsive, and theranostic 19F magnetic resonance imaging probes. Bioconjugate Chemistry, 2019, 30(10): 2502-2518.
DOI URL |
| [29] |
ZHAO X, DUAN G, WU K, et al. Intelligent metamaterials based on nonlinearity for magnetic resonance imaging. Advanced Materials, 2019, 31(49): 1905461-7.
DOI URL |
| [30] |
DELCASSIAN D, LUZHANSKY I, SPANOUDAKI V, et al. Magnetic retrieval of encapsulated beta cell transplants from diabetic mice using dual-function MRI visible and retrievable microcapsules. Advanced Materials, 2020, 32(16): 1904502-10.
DOI URL |
| [31] |
ZHANG J, YUAN Y, GAO M, et al. Carbon dots as a new class of diamagnetic chemical exchange saturation transfer (diacest) MRI contrast agents. Angewandte Chemie International Edition, 2019, 58(29): 9871-9875.
DOI URL |
| [32] |
ZHANG P, HOU Y, ZENG J, et al. Coordinatively unsaturated Fe3+ based activatable probes for enhanced MRI and therapy of tumors. Angewandte Chemie International Edition, 2019, 58(32): 11088- 11096.
DOI URL |
| [33] |
DAI C, CHEN Y, JING X X, et al. Two-dimensional tantalum carbide (MXenes) composite nanosheets for multiple imaging- guided photothermal tumor ablation. ACS Nano, 2017, 11(12): 12696-12712.
DOI URL |
| [34] |
DAI C, LIN H, XU G, et al. Biocompatible 2D titanium carbide (MXenes) composite nanosheets for pH-responsive MRI-guided tumor hyperthermia. Chemistry of Materials, 2017, 29(20): 8637-8652.
DOI URL |
| [35] |
LIU Z, LIN H, ZHAO M L, et al. 2D superparamagnetic tantalum carbide composite MXenes for efficient breast-cancer theranostics. Theranostics, 2018, 8(6): 1648-1664.
DOI PMID |
| [36] |
LIU Z, ZHAO M L, LIN H, et al. 2D magnetic titanium carbide MXene for cancer theranostics. Journal of Materials Chemistry B, 2018, 6(21): 3541-3548.
DOI PMID |
| [37] | CAO Y, WU T, ZHANG K, et al. Engineered exosome-mediated near-infrared-II region V2C quantum dot delivery for nucleus-target low-temperature photothermal therapy. ACS Nano, 2019, 13(2): 1499-1510. |
| [38] |
BAR-ZION A, NOURMAHNAD A, MITTELSTEIN D R, et al. Acoustically triggered mechanotherapy using genetically encoded gas vesicles. Nature Nanotechnology, 2021, 16(12): 1403-1412.
DOI URL |
| [39] |
LIU Y, BHATTARAI P, DAI Z, et al. Photothermal therapy and photoacoustic imaging via nanotheranostics in fighting cancer. Chemical Society Reviews, 2019, 48(7): 2053-2108.
DOI URL |
| [40] |
LI X, CAI Z, JIANG L P, et al. Metal-ligand coordination nanomaterials for biomedical imaging. Bioconjugate Chemistry, 2019, 31(2): 332-339.
DOI URL |
| [41] |
MENG Z, ZHOU X, SHE J, et al. Ultrasound-responsive conversion of microbubbles to nanoparticles to enable background-free in vivo photoacoustic imaging. Nano Letters, 2019, 19(11): 8109-8117.
DOI URL |
| [42] |
CHEN Y S, ZHAO Y, YOON S J, et al. Miniature gold nanorods for photoacoustic molecular imaging in the second near-infrared optical window. Nature Nanotechnology, 2019, 14(5): 465-472.
DOI URL |
| [43] |
KIM J, CHHOUR P, HSU J, et al. Use of nanoparticle contrast agents for cell tracking with computed tomography. Bioconjugate Chemistry, 2017, 28(6): 1581-1597.
DOI PMID |
| [44] |
ZHOU B G, YIN H H, DONG C H, et al. Biodegradable and excretable 2D W1.33C i-MXene with vacancy ordering for theory- oriented cancer nanotheranostics in near-infrared biowindow. Advanced Science, 2021, 8(24): 2101043-13.
DOI URL |
| [45] |
XU J, SHI R, CHEN G, et al. All-in-one theranostic nanomedicine with ultrabright second near-infrared emission for tumor- modulated bioimaging and chemodynamic/photodynamic therapy. ACS Nano, 2020, 14(8): 9613-9625.
DOI URL |
| [46] |
JUNG H S, VERWILST P, SHARMA A, et al. Organic molecule- based photothermal agents: an expanding photothermal therapy universe. Chemical Society Reviews, 2018, 47(7): 2280-2297.
DOI URL |
| [47] | WANG H, CHANG J, SHI M, et al. A dual-targeted organic photothermal agent for enhanced photothermal therapy. Angewandte Chemie International Edition, 2019, 131(4): 1069-1073. |
| [48] |
YU Z, HU W, ZHAO H, et al. Generating new cross-relaxation pathways by coating prussian blue on NaNdF4 to fabricate enhanced photothermal agents. Angewandte Chemie International Edition, 2019, 58(25): 8536-8540.
DOI URL |
| [49] |
JIANG Y, LI J, ZHEN X, et al. Dual-peak absorbing semiconducting copolymer nanoparticles for first and second near-infrared window photothermal therapy: a comparative study. Advanced Materials, 2018, 30(14): 1705980-7.
DOI URL |
| [50] | ZHEN X, XIE C, PU K. Temperature-correlated afterglow of a semiconducting polymer nanococktail for imaging-guided photothermal therapy. Angewandte Chemie International Edition, 2018, 130(15): 4002-4006. |
| [51] |
SMITH A M, MANCINI M C, NIE S. Second window for in vivo imaging. Nature Nanotechnology, 2009, 4(11): 710-711.
DOI URL |
| [52] |
ZHOU J, JIANG Y, HOU S, et al. Compact plasmonic blackbody for cancer theranosis in the near-infrared II window. ACS Nano, 2018, 12(3): 2643-2651.
DOI PMID |
| [53] |
CHENG Y, YANG F, XIANG G, et al. Ultrathin tellurium oxide/ ammonium tungsten bronze nanoribbon for multimodality imaging and second near-infrared region photothermal therapy. Nano Letters, 2019, 19(2): 1179-1189.
DOI URL |
| [54] |
CUI J, JIANG R, GUO C, et al. Fluorine grafted Cu7S4-Au heterodimers for multimodal imaging guided photothermal therapy with high penetration depth. Journal of the American Chemical Society, 2018, 140(18): 5890-5894.
DOI URL |
| [55] |
SUN S, SONG Y, CHEN J, et al. NIR-I and NIR-II irradiation tumor ablation using NbS2 nanosheets as the photothermal agent. Nanoscale, 2021, 13(43): 18300-18310.
DOI URL |
| [56] |
XIANG H J, CHEN Y. Energy-converting nanomedicine. Small, 2019, 15(13): 1805339-31.
DOI URL |
| [57] |
HU H, QIAN X Q, CHEN Y. Microalgae-enabled photosynthetic alleviation of tumor hypoxia for enhanced nanotherapies. Science Bulletin, 2020, 65(22): 1869-1871.
DOI URL |
| [58] |
CHEN B D, XIANG H J, PAN S S, et al. Advanced theragenerative biomaterials with therapeutic and regeneration multifunctionality. Advanced Functional Materials, 2020, 30(34): 2002621-27.
DOI URL |
| [59] |
FENG W, CHEN Y. Chemoreactive nanomedicine. Journal of Materials Chemistry B, 2020, 8(31): 6753-6764.
DOI PMID |
| [60] |
LUCKY S S, SOO K C, ZHANG Y. Nanoparticles in photodynamic therapy. Chemical Reviews, 2015, 115(4): 1990-2042.
DOI PMID |
| [61] | CHANG M, FENG W, DING L, et al. Persistent luminescence phosphor as in-vivo light source for tumoral cyanobacterial photosynthetic oxygenation and photodynamic therapy. Bioactive Materials, 2022, 10: 131-144. |
| [62] | ZHOU L, DONG C, DING L, et al. Targeting ferroptosis synergistically sensitizes apoptotic sonodynamic anti-tumor nanotherapy. Nano Today, 2021, 39: 101212-12. |
| [63] |
SHEN Y, CHEN L, GUAN X, et al. Tailoring chemoimmunostimulant bioscaffolds for inhibiting tumor growth and metastasis after incomplete microwave ablation. ACS Nano, 2021, 15(12): 20414-20429.
DOI PMID |
| [64] |
MELLMAN I, COUKOS G, DRANOFF G. Cancer immunotherapy comes of age. Nature, 2011, 480(7378): 480-489.
DOI URL |
| [65] |
RIBAS A, WOLCHOK J D. Cancer immunotherapy using checkpoint blockade. Science, 2018, 359(6382): 1350-1355.
DOI URL |
| [66] |
MAHONEY K M, RENNERT P D, FREEMAN G J. Combination cancer immunotherapy and new immunomodulatory targets. Nature Reviews Drug Discovery, 2015, 14(8): 561-584.
DOI PMID |
| [67] |
VANNEMAN M, DRANOFF G. Combining immunotherapy and targeted therapies in cancer treatment. Nature Reviews Cancer, 2012, 12(4): 237-251.
DOI PMID |
| [68] |
SANG W, ZHANG Z, DAI Y, et al. Recent advances in nanomaterial-based synergistic combination cancer immunotherapy. Chemical Society Reviews, 2019, 48(14): 3771-3810.
DOI PMID |
| [69] |
SHIELDS IV CW, WANG L W, EVANS M A, et al. Materials for immunotherapy. Advanced Materials, 2020, 32(13): 1901633-56.
DOI URL |
| [70] |
NAM J, SON S, PARK K S, et al. Cancer nanomedicine for combination cancer immunotherapy. Nature Reviews Materials, 2019, 4(6): 398-414.
DOI |
| [71] | SONG W, MUSETTI S N, HUANG L. Nanomaterials for cancer immunotherapy. Biomaterials, 2017, 148: 16-30. |
| [72] |
CHEUNG A S, MOONEY D J. Engineered materials for cancer immunotherapy. Nano Today, 2015, 10(4): 511-531.
PMID |
| [73] |
LIU Y, WANG L, SONG Q, et al. Intrapleural nano-immunotherapy promotes innate and adaptive immune responses to enhance anti- PD-L1 therapy for malignant pleural effusion. Nature Nanotechnology, 2022, 17(2): 206-216.
DOI URL |
| [74] |
ZHENG Y, LI X, DONG C, et al. Ultrasound-augmented nanocatalytic ferroptosis reverses chemotherapeutic resistance and induces synergistic tumor nanotherapy. Advanced Functional Materials, 2022, 32(4): 2107529-17.
DOI URL |
| [75] |
XU W, DONG C, HU H, et al. Engineering Janus chemoreactive nanosonosensitizers for bilaterally augmented sonodynamic and chemodynamic cancer nanotherapy. Advanced Functional Materials, 2021, 31(37): 2103134-13.
DOI URL |
| [76] | XIANG H, YOU C, LIU W, et al. Chemotherapy-enabled /augmented cascade catalytic tumor-oxidative nanotherapy. Biomaterials, 2021, 277: 121071-12. |
| [77] |
WANG H X, LI M, LEE C M, et al. CRISPR/Cas9-based genome editing for disease modeling and therapy: challenges and opportunities for nonviral delivery. Chemical Reviews, 2017, 117(15): 9874-9906.
DOI URL |
| [78] |
LYU Y, HE S S, LI J C, et al. A photolabile semiconducting polymer nanotransducer for near-infrared regulation of CRISPR/ Cas9 gene editing. Angewandte Chemie International Edition, 2019, 58(50): 18197-18201.
DOI URL |
| [79] |
CHENG Q, WEI T, FARBIAK L, et al. Selective organ targeting (SORT) nanoparticles for tissue-specific mRNA delivery and CRISPR-Cas gene editing. Nature Nanotechnology, 2020, 15(4): 313-320.
DOI PMID |
| [80] |
YIN H, ZHOU B, DONG C, et al. CRISPR/Cas9-2D silicene gene-editing nanosystem for remote NIR-II-induced tumor microenvironment reprogramming and augmented photonic tumor ablation. Advanced Functional Materials, 2021, 31(50): 2107093-12.
DOI URL |
| [81] |
YIN H, SUN L, PU Y, et al. Ultrasound-controlled CRISPR/Cas9 system augments sonodynamic therapy of hepatocellular carcinoma. ACS Central Science, 2021, 7(12): 2049-2062.
DOI PMID |
| [82] |
LI M, MA H, HAN F, et al. Microbially catalyzed biomaterials for bone regeneration. Advanced Materials, 2021, 33(49): 2104829-13.
DOI URL |
| [83] | ZHOU Y, WU C, CHANG J. Bioceramics to regulate stem cells and their microenvironment for tissue regeneration. Materials Today, 2019, 24: 41-56. |
| [84] |
LI T, CHANG J, ZHU Y, et al. 3D printing of bioinspired biomaterials for tissue regeneration. Advanced Healthcare Materials, 2020, 9(23): 2000208-17.
DOI URL |
| [85] | STEVENS M M. Biomaterials for bone tissue engineering. Materials Today, 2008, 11(5): 18-25. |
| [86] |
DISCHER D E, MOONEY D J, ZANDSTRA P W. Growth factors, matrices, and forces combine and control stem cells. Science, 2009, 324(5935): 1673-1677.
DOI URL |
| [87] |
GEIGER B, SPATZ J P, BERSHADSKY A D. Environmental sensing through focal adhesions. Nature Reviews Molecular Cell Biology, 2009, 10(1): 21-33.
DOI PMID |
| [88] |
WANG L, YANG Q, HUO M, et al. Engineering single-atomic iron-catalyst-integrated 3D-printed bioscaffolds for osteosarcoma destruction with antibacterial and bone defect regeneration bioactivity. Advanced Materials, 2021, 33(31): 2100150-12.
DOI URL |
| [89] |
SOMMARIVA M, LE NOCI V, BIANCHI F, et al. The lung microbiota: role in maintaining pulmonary immune homeostasis and its implications in cancer development and therapy. Cellular and Molecular Life Sciences, 2020, 77(14): 2739-2749.
DOI PMID |
| [90] |
YANG W, PETERS J I, WILLIAMS R O. Inhaled nanoparticles-a current review. International Journal of Pharmaceutics, 2008, 356(1): 239-247.
DOI URL |
| [91] |
MYERSON J W, PATEL P N, RUBEY K M, et al. Supramolecular arrangement of protein in nanoparticle structures predicts nanoparticle tropism for neutrophils in acute lung inflammation. Nature Nanotechnology, 2022, 17(1): 86-97.
DOI URL |
| [92] | VASARMIDI E, TSITOURA E, SPANDIDOS D A, et al. Pulmonary fibrosis in the aftermath of the COVID-19 era. Experimental and Therapeutic Medicine, 2020, 20(3): 2557-2560. |
| [93] |
CRISAN-DABIJA R, PAVEL C A, POPA I V, et al. “A chain only as strong as its weakest link”: an up-to-date literature review on the bidirectional interaction of pulmonary fibrosis and COVID-19. Journal of Proteome Research, 2020, 19(11): 4327-4338.
DOI URL |
| [94] |
LEDERER D J, MARTINEZ F J. Idiopathic pulmonary fibrosis. New England Journal of Medicine, 2018, 378(19): 1811-1823.
DOI URL |
| [95] |
RICHELDI L, COLLARD H R, JONES M G. Idiopathic pulmonary fibrosis. Lancet, 2017, 389(10082): 1941-1952.
DOI PMID |
| [96] | MERKT W, BUENO M, MORA AL, et al. Senotherapeutics: targeting senescence in idiopathic pulmonary fibrosis. Seminars in Cell & Developmental Biology, 2020, 101: 104-110. |
| [97] | MALSIN E S, KAMP D W. The mitochondria in lung fibrosis: Friend or foe? Translational Research, 2018, 202: 1-23. |
| [98] |
YU G, TZOUVELEKIS A, WANG R, et al. Thyroid hormone inhibits lung fibrosis in mice by improving epithelial mitochondrial function. Nature Medicine, 2018, 24(1): 39-49.
DOI PMID |
| [99] | SALEH J, PEYSSONNAUX C, SINGH K K, et al. Mitochondria and microbiota dysfunction in COVID-19 pathogenesis. Mitochondrion, 2020, 54: 1-7. |
| [100] |
HUANG T, ZHANG T, JIANG X, et al. Iron oxide nanoparticles augment the intercellular mitochondrial transfer-mediated therapy. Science Advances, 2021, 7(40): eabj0534-15.
DOI URL |
| [101] |
FENG W, HAN X G, HU H, et al. 2D vanadium carbide MXenzyme to alleviate ROS-mediated inflammatory and neurodegenerative diseases. Nature Communications, 2021, 12(1): 2203-16.
DOI PMID |
| [102] |
WANG K, ZHANG Y, MAO W, et al. Engineering ultrasmall ferroptosis-targeting and reactive oxygen/nitrogen species- scavenging nanozyme for alleviating acute kidney injury. Advanced Functional Materials, 2022, 32(10): 2109221-13.
DOI URL |
| [103] |
TANG Z, HUO M, JU Y, et al. Nanoprotection against retinal pigment epithelium degeneration via ferroptosis inhibition. Small Methods, 2021, 5(12): 2100848-14.
DOI URL |
| [104] |
WU J, YUK H, SARRAFIAN TIFFANY L, et al. An off-the-shelf bioadhesive patch for sutureless repair of gastrointestinal defects. Science Translational Medicine, 14(630): eabh2857-13.
DOI URL |
| [105] |
KIM S J, CHOI S J, JANG J S, et al. Innovative nanosensor for disease diagnosis. Accounts of Chemical Research, 2017, 50(7): 1587-1596.
DOI URL |
| [106] |
TANG Z M, KONG N, ZHANG X C, et al. A materials-science perspective on tackling COVID-19. Nature Reviews Materials, 2020, 5(11): 847-860.
DOI URL |
| [107] |
FAROKHZAD N, TAO W. Materials chemistry-enabled platforms in detecting sexually transmitted infections: progress towards point-of-care tests. Trends in Chemistry, 2021, 3(7): 589-602.
DOI URL |
| [108] |
GONG M M, SINTON D. Turning the page: advancing paper-based microfluidics for broad diagnostic application. Chemical Reviews, 2017, 117(12): 8447-8480.
DOI PMID |
| [109] |
HUANG X, LIU Y, YUNG B, et al. Nanotechnology-enhanced no-wash biosensors for in vitro diagnostics of cancer. ACS Nano, 2017, 11(6): 5238-5292.
DOI URL |
| [110] |
WANG C, QI B, LIN M, et al. Continuous monitoring of deep-tissue haemodynamics with stretchable ultrasonic phased arrays. Nature Biomedical Engineering, 2021, 5(7): 749-758.
DOI PMID |
| [111] |
CHAIBUN T, PUENPA J, NGAMDEE T, et al. Rapid electrochemical detection of coronavirus SARS-Cov-2. Nature Communications, 2021, 12(1): 802-10.
DOI PMID |
| [112] |
GATES B. Responding to COVID-19-a once-in-a-century pandemic? New England Journal of Medicine, 2020, 382(18): 1677-1679.
DOI URL |
| [113] |
LAURING A S, FRYDMAN J, ANDINO R. The role of mutational robustness in RNA virus evolution. Nature Reviews Microbiology, 2013, 11(5): 327-336.
DOI PMID |
| [114] |
SEO G, LEE G, KIM M J, et al. Rapid detection of COVID-19 causative virus (SARS-Cov-2) in human nasopharyngeal swab specimens using field-effect transistor-based biosensor. ACS Nano, 2020, 14(4): 5135-5142.
DOI PMID |
| [115] |
XIAO G, HE J, CHEN X, et al. A wearable, cotton thread/paper- based microfluidic device coupled with smartphone for sweat glucose sensing. Cellulose, 2019, 26(7): 4553-4562.
DOI URL |
| [116] |
LI T, CHEN L, YANG X, et al. A flexible pressure sensor based on an MXene-textile network structure. Journal of Materials Chemistry C, 2019, 7(4): 1022-1027.
DOI |
| [117] |
KALELKAR P P, RIDDICK M, GARCIA A J. Biomaterial-based antimicrobial therapies for the treatment of bacterial infections. Nature Reviews Materials, 2022, 7(1): 39-54.
DOI URL |
| [118] |
LI J F, LI Z Y, LIU X M, et al. Interfacial engineering of Bi2S3/Ti3C2Tx MXene based on work function for rapid photo- excited bacteria-killing. Nature Communications, 2021, 12(1): 1224-10.
DOI URL |
| [119] |
DURÁN N, DURÁN M, DE JESUS MB, et al. Silver nanoparticles: a new view on mechanistic aspects on antimicrobial activity. Nanomedicine: Nanotechnology, Biology and Medicine, 2016, 12(3): 789-799.
DOI URL |
| [120] |
PANÁČEK A, KVÍTEK L, SMÉKALOVÁ M, et al. Bacterial resistance to silver nanoparticles and how to overcome it. Nature Nanotechnology, 2018, 13(1): 65-71.
DOI PMID |
| [121] |
STABRYLA L M, JOHNSTON K A, DIEMLER N A, et al. Role of bacterial motility in differential resistance mechanisms of silver nanoparticles and silver ions. Nature Nanotechnology, 2021, 16(9): 996-1003.
DOI URL |
| [122] |
LI B, WANG W, SONG W, et al. Antiviral and anti-inflammatory treatment with multifunctional alveolar macrophage-like nanoparticles in a surrogate mouse model of COVID-19. Advanced Science, 2021, 8(13): 2003556-14.
DOI URL |
| [123] |
VALLIERES C, HOOK A L, HE Y, et al. Discovery of (meth)acrylate polymers that resist colonization by fungi associated with pathogenesis and biodeterioration. Science Advances, 2020, 6(23): eaba6574-12.
DOI URL |
| [1] | 丁玲, 蒋瑞, 唐子龙, 杨运琼. MXene材料的纳米工程及其作为超级电容器电极材料的研究进展[J]. 无机材料学报, 2023, 38(6): 619-633. |
| [2] | 杨卓, 卢勇, 赵庆, 陈军. X射线衍射Rietveld精修及其在锂离子电池正极材料中的应用[J]. 无机材料学报, 2023, 38(6): 589-605. |
| [3] | 陈强, 白书欣, 叶益聪. 热管理用高导热碳化硅陶瓷基复合材料研究进展[J]. 无机材料学报, 2023, 38(6): 634-646. |
| [4] | 林俊良, 王占杰. 铁电超晶格的研究进展[J]. 无机材料学报, 2023, 38(6): 606-618. |
| [5] | 牛嘉雪, 孙思, 柳鹏飞, 张晓东, 穆晓宇. 铜基纳米酶的特性及其生物医学应用[J]. 无机材料学报, 2023, 38(5): 489-502. |
| [6] | 苑景坤, 熊书锋, 陈张伟. 聚合物前驱体转化陶瓷增材制造技术研究趋势与挑战[J]. 无机材料学报, 2023, 38(5): 477-488. |
| [7] | 杜剑宇, 葛琛. 光电人工突触研究进展[J]. 无机材料学报, 2023, 38(4): 378-386. |
| [8] | 杨洋, 崔航源, 祝影, 万昌锦, 万青. 柔性神经形态晶体管研究进展[J]. 无机材料学报, 2023, 38(4): 367-377. |
| [9] | 游钧淇, 李策, 杨栋梁, 孙林锋. 氧化物双介质层忆阻器的设计及应用[J]. 无机材料学报, 2023, 38(4): 387-398. |
| [10] | 林思琪, 李艾燃, 付晨光, 李荣斌, 金敏. Zintl相Mg3X2(X=Sb, Bi)基晶体生长及热电性能研究进展[J]. 无机材料学报, 2023, 38(3): 270-279. |
| [11] | 齐占国, 刘磊, 王守志, 王国栋, 俞娇仙, 王忠新, 段秀兰, 徐现刚, 张雷. GaN单晶的HVPE生长与掺杂进展[J]. 无机材料学报, 2023, 38(3): 243-255. |
| [12] | 张超逸, 唐慧丽, 李宪珂, 王庆国, 罗平, 吴锋, 张晨波, 薛艳艳, 徐军, 韩建峰, 逯占文. 新型GaN与ZnO衬底ScAlMgO4晶体的研究进展[J]. 无机材料学报, 2023, 38(3): 228-242. |
| [13] | 陈昆峰, 胡乾宇, 刘锋, 薛冬峰. 多尺度晶体材料的原位表征技术与计算模拟研究进展[J]. 无机材料学报, 2023, 38(3): 256-269. |
| [14] | 谢兵, 蔡金峡, 王铜铜, 刘智勇, 姜胜林, 张海波. 高储能密度聚合物基多层复合电介质的研究进展[J]. 无机材料学报, 2023, 38(2): 137-147. |
| [15] | 冯静静, 章游然, 马名生, 陆毅青, 刘志甫. 冷烧结技术的研究现状及发展趋势[J]. 无机材料学报, 2023, 38(2): 125-136. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||