无机材料学报 ›› 2022, Vol. 37 ›› Issue (8): 883-890.DOI: 10.15541/jim20220097
所属专题: 【能源环境】金属有机框架材料
张笑宇( ), 刘永盛, 李然, 李耀刚, 张青红, 侯成义, 李克睿(
), 刘永盛, 李然, 李耀刚, 张青红, 侯成义, 李克睿( ), 王宏志(
), 王宏志( )
)
收稿日期:2022-02-28
修回日期:2022-05-31
出版日期:2022-08-20
网络出版日期:2022-06-03
通讯作者:
王宏志, 教授. E-mail: wanghz@dhu.edu.cn;作者简介:张笑宇(1998-), 男, 硕士研究生. E-mail: dhuzxyu@163.com
基金资助:
ZHANG Xiaoyu( ), LIU Yongsheng, LI Ran, LI Yaogang, ZHANG Qinghong, HOU Chengyi, LI Kerui(
), LIU Yongsheng, LI Ran, LI Yaogang, ZHANG Qinghong, HOU Chengyi, LI Kerui( ), WANG Hongzhi(
), WANG Hongzhi( )
)
Received:2022-02-28
Revised:2022-05-31
Published:2022-08-20
Online:2022-06-03
Contact:
WANG Hongzhi, professor. E-mail: wanghz@dhu.edu.cn;About author:ZHANG Xiaoyu(1998-), male, Master candidate. E-mail: dhuzxyu@163.com
Supported by:摘要:
室温离子液体具有宽电化学窗口和良好的环境稳定性, 是电致变色器件的理想电解质。然而传统电致变色材料的晶格间隙较窄, 限制了离子液体中大尺寸离子的扩散, 且大离子反复脱/嵌会破坏传统电致变色材料的结构, 导致性能衰减。金属有机框架材料(MOFs)是一种具有拓扑结构的多孔晶态材料, 有望为离子液体中大尺寸离子的传输提供通道。本工作在导电玻璃表面制备了三亚苯类Cu3(HHTP)2 (HHTP=2,3,6,7,10,11-六羟基三苯并菲) MOF薄膜, 并研究了Cu3(HHTP)2薄膜在离子液体电解质中电化学和电致变色行为和性能。结果表明, 相对于传统的LiClO4/PC和NaClO4/PC电解质, Cu3(HHTP)2薄膜在离子液体[EMIm]BF4中表现出更低的接触电阻和更高的离子扩散效率, 电极的着色/褪色速度得到了显著提高(着色时间由10.3 s缩短至8.0 s, 褪色时间由23.6 s缩短至5.2 s)。同时, Cu3(HHTP)2薄膜在[EMIm]BF4中也具有更高的光调制范围和着色效率。这项工作展现出MOFs/离子液体电化学体系在电致变色领域中的潜在应用价值。
中图分类号:
张笑宇, 刘永盛, 李然, 李耀刚, 张青红, 侯成义, 李克睿, 王宏志. 基于Cu3(HHTP)2薄膜的离子液体电致变色电极[J]. 无机材料学报, 2022, 37(8): 883-890.
ZHANG Xiaoyu, LIU Yongsheng, LI Ran, LI Yaogang, ZHANG Qinghong, HOU Chengyi, LI Kerui, WANG Hongzhi. Cu3(HHTP)2 Film-based Ionic-liquid Electrochromic Electrode[J]. Journal of Inorganic Materials, 2022, 37(8): 883-890.
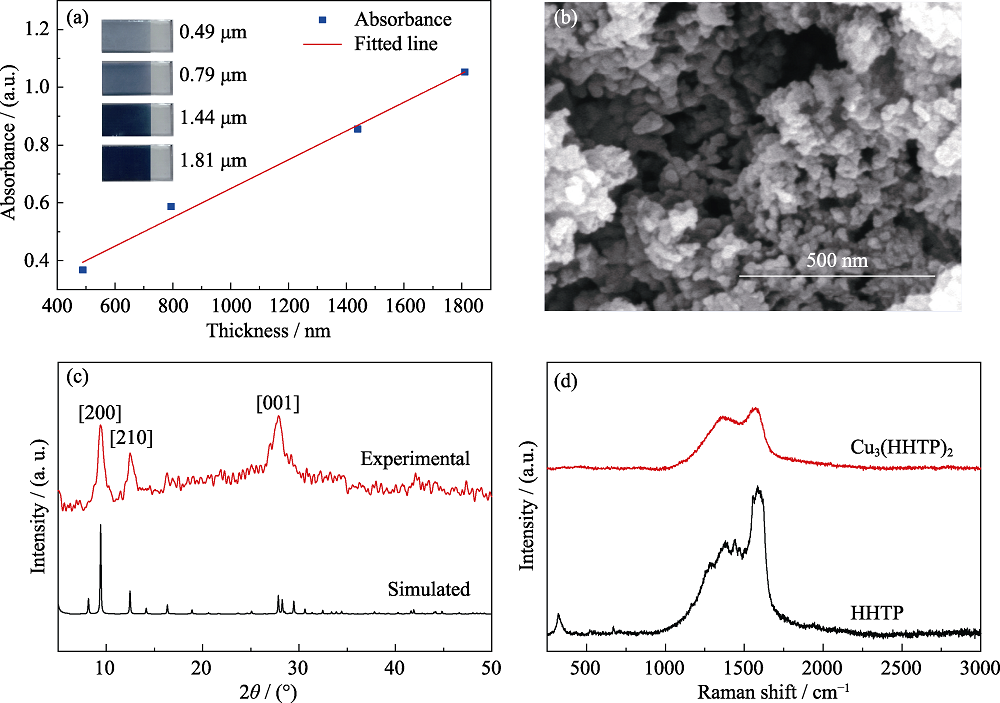
图1 (a) Cu3(HHTP)2薄膜的表征结果
Fig. 1 Characterization of Cu3(HHTP)2 films (a) Change of absorbance at 800 nm wavelength with film thickness, inset showing the pictures of Cu3(HHTP)2 films obtained in different growth-cycles; (b) Surface SEM image of the Cu3(HHTP)2 film obtained from 20 cycles; (c) XRD patterns of Cu3(HHTP)2; (d) Raman spectra of Cu3(HHTP)2 and HHTP ligand

图2 Cu3(HHTP)2的XPS谱图和孔径分布图
Fig. 2 XPS spectrum and poresize distribution of Cu3(HHTP)2 (a) XPS full spectrum; (b) High resolution XPS spectrum of Cu2p3/2; (c) Pore size distribution diagram with inset showing N2 adsorption isotherm curves for Cu3(HHTP)2 powders measured at 77 K
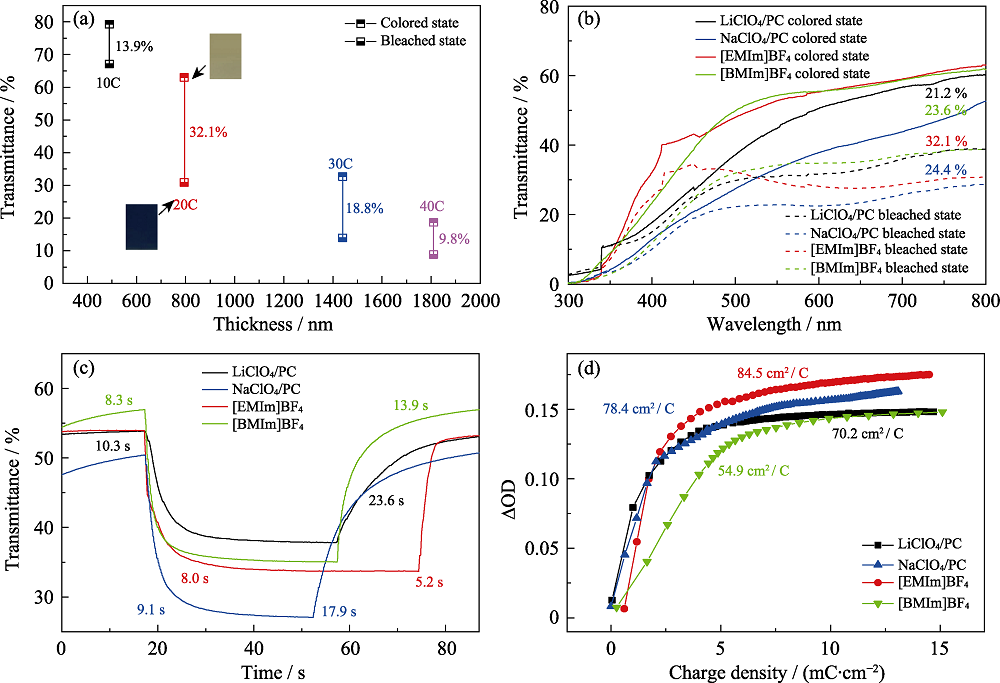
图3 (a)不同厚度的Cu3(HHTP)2薄膜在[EMIm]BF4中-0.9和0.4 V的恒定电压下, 在800 nm波长处的透过率变化图谱(插图为20C薄膜在-0.9 和0.4 V下的照片); (b) 20C薄膜在不同电解质中, -0.9和0.4 V下的紫外-可见透过光图谱(300~800 nm); (c) 20C薄膜在不同电解质中, 在波长800 nm处的透过光谱时间响应图; (d) 20C薄膜分别在LiClO4/PC、NaClO4/PC、[EMIm]BF4和[BMIm]BF4溶液中800 nm波长处的着色效率
Fig. 3 (a) Transmittance at 800 nm wavelength for Cu3(HHTP)2 films with different thicknesses at constant voltages of -0.9 and 0.4 V in [EMIm]BF4 with inset photos showing 20C film at -0.9 V and 0.4 V ; (b) UV-Vis transmission spectra of 20C films measured in various electrolytes at wavelength from 300 to 800 nm; (c) Temporal response of the transmittance of 20C films measured in various electrolytes; (d) Coloring efficiencies of 20C films in various electrolytes, respectively
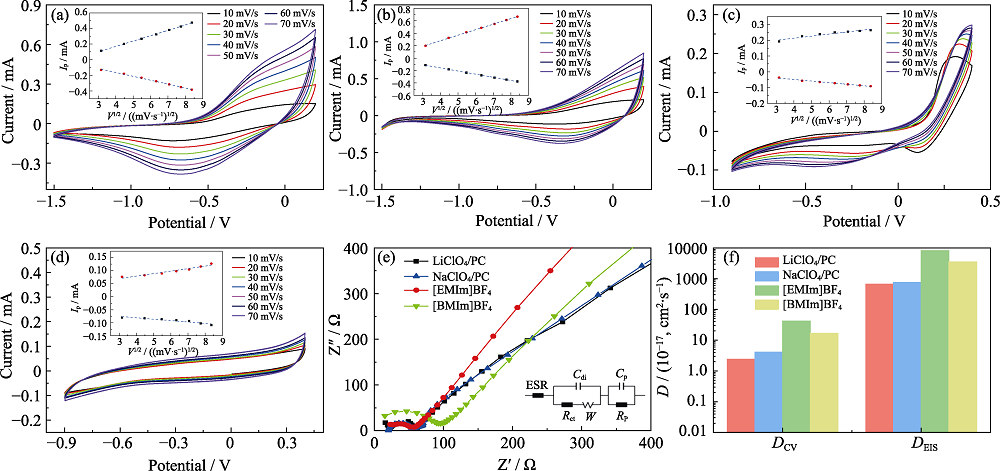
图4 20C薄膜在LiClO4/PC(a)、NaClO4/PC(b)、[EMIm]BF4(c)和[BMIm]BF4(d)中10~70 mV∙s-1扫描速率下的循环伏安曲线(插图为不同扫速下峰值电流(ip)与扫描速率平方根(V1/2)的函数; (e) 20C薄膜分别在不同电解质中的Nyquist阻抗数据(点)和相应拟合结果(线)(插图为对应的等效电路); (f)从电化学阻抗谱和循环伏安测试中计算得出20C薄膜在不同电解质中的扩散系数
Fig. 4 Cyclic voltammetry curves of 20C films at scan rates from 10 to 70 mV∙s-1 in (a) LiClO4/PC, (b) NaClO4/PC solution, (c) [EMIm]BF4, and (d) [BMIm]BF4 with inset showing peak current at different scan rates (ip) as a function of square root of the scan rate (V1/2)); (e) Nyquist impedance data (dots) and corresponding fitting results (lines) of 20C films in various electrolytes, respectively with inset showing corresponding equivalent circuit; (f) Calculated diffusion coefficients of 20C films in various electrolytes from electrochemical impedance spectroscopy and cyclic voltammetry, respectively

图5 Cu3(HHTP)2基电致变色器件在(a)初始态和(b)透明态的照片; (c)器件在+3和-3 V电压下的紫外-可见光透射图谱
Fig. 5 Photos of (a) bleaching state and (b) coloring state of Cu3(HHTP)2 EC device, and (c) UV-Vis transmission spectra of Cu3(HHTP)2 EC devices at voltages of +3 and -3 V
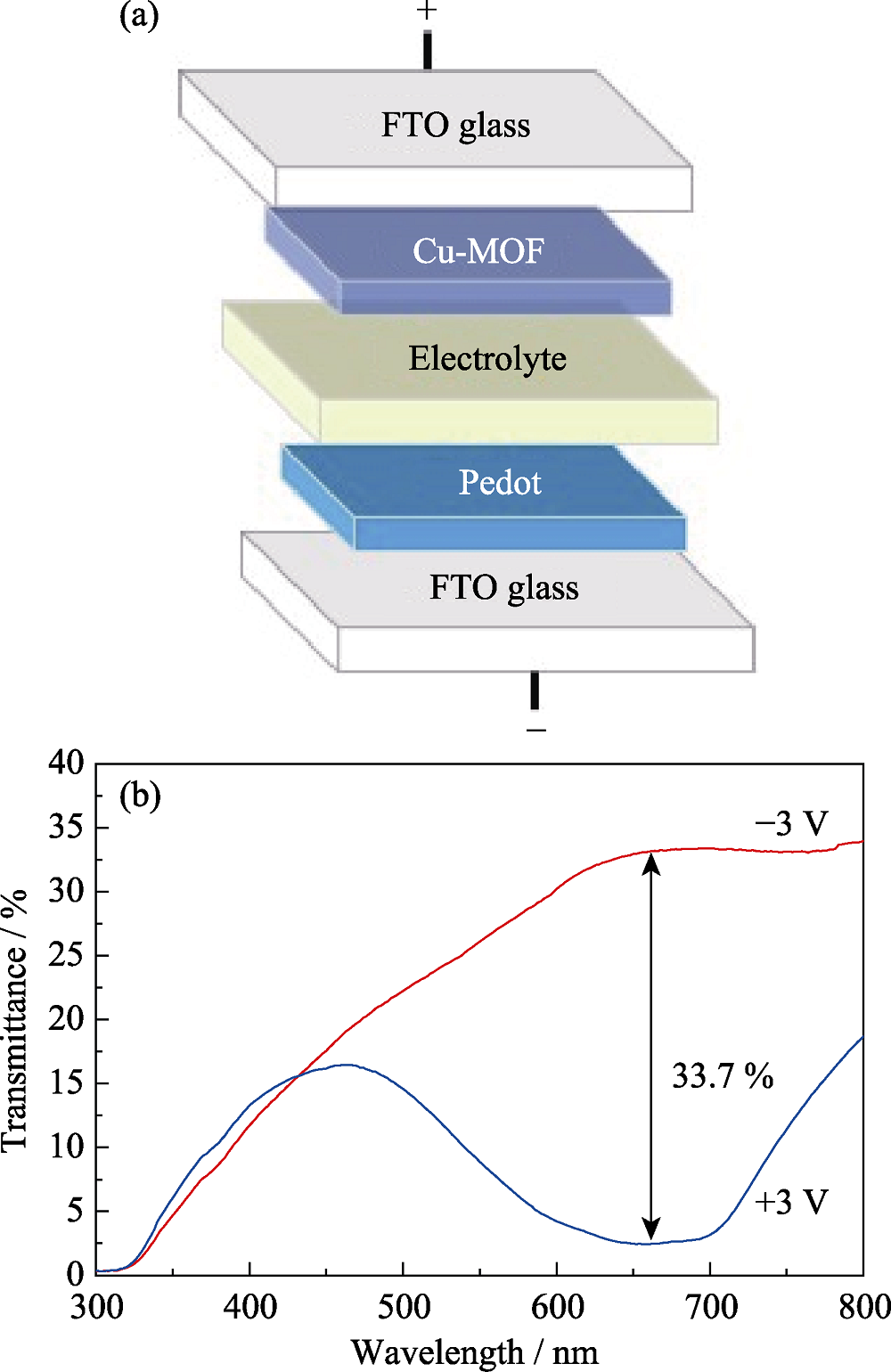
图6 (a)基于Cu3(HHTP)2和PEDOT电致变色全器件结构示意图和(b)电致变色全器件在+3和-3 V电压下的紫外-可见光透射图谱
Fig. 6 (a) Structure diagram of Cu3(HHTP)2 and poly (3,4-ethylene dioxythiophene) (PEDOT) electrochromic multiple device, and (b) UV-Vis transmission spectra of multiple devices at voltages of +3 and -3 V
| [1] |
XU C, LIU L, LEGENSKI S, et al. Switchable window based on electrochromic polymers. Journal of Materials Research, 2004, 19(7): 2072-2080.
DOI URL |
| [2] |
WU X, ZHENG J, XU C. A newly-designed self-powered electrochromic window. Science China Chemistry, 2016, 60(1): 84-89.
DOI URL |
| [3] |
WADE C R, LI M, DINCĂ M. Facile deposition of multicolored electrochromic metal-organic framework thin films. Angewandte Chemie International Edition, 2013, 52: 13377-13381.
DOI URL |
| [4] |
WADE C R, LI M, DINCĂ M. Transparent-to-dark electrochromic behavior in naphthalene-diimide-based mesoporous MOF-74 analogs. Chem, 2016, 1(11): 264-272.
DOI URL |
| [5] |
RIERA M, LAMBROS E, NGUYEN T, et al. Low-order many-body interactions determine the local structure of liquid water. Chemical Science, 2019, 10(35): 8211-8218.
DOI URL |
| [6] |
LI R, LI K, WANG G, et al. Ion-transport design for high- performance Na+-based electrochromics. ACS Nano, 2018, 12: 3759-3768.
DOI URL |
| [7] |
MUKHIYA T, OJHA G, DAHAL B, et al. Designed assembly of porous cobalt oxide/carbon nanotentacles on electrospun hollow carbon nanofibers network for supercapacitor. ACS Applied Energy Materials, 2020, 3(4): 3435-3444.
DOI URL |
| [8] |
QIU X, WANG N, DONG X, et al. A high-voltage Zn-organic battery using a nonflammable organic electrolyte. Angewandte Chemie International Edition, 2021, 60: 21025-21032.
DOI URL |
| [9] |
KIM J, LEE J, YOU J, et al. Conductive polymers for next- generation energy storage systems: recent progress and new functions. Materials Horizons, 2016, 3(6): 517-535.
DOI URL |
| [10] |
CHEN J, NAVEED A, NULI Y, et al. Designing an intrinsically safe organic electrolyte for rechargeable batteries. Energy Storage Materials, 2020, 31: 382-400.
DOI URL |
| [11] |
DANG L, WICK C. Anion effects on interfacial absorption of gases in ionic liquids: a molecular dynamics study. Journal of Physical Chemistry B, 2011, 115(21): 6964-6970.
DOI URL |
| [12] | WILKES J, ZAWOROTKO M. Air and water stable 1-ethyl-3- methylimidazolium based ionic liquids. Chemical Society Chemical Communications, 1992, 13: 965-967. |
| [13] |
CHEN Y, ZHANG X, ZHANG D, et al. High performance supercapacitors based on reducedgraphene oxide in aqueous and ionic liquid electrolytes. Carbon, 2011, 49: 573-580.
DOI URL |
| [14] |
BALDUCCI A, DUGAS R, TABERNA P, et al. High temperature carbon-carbon supercapacitor using ionic liquid as electrolyte. Journal of Power Sources, 2007, 165: 922-927.
DOI URL |
| [15] |
JIN J, WEN Z, LIANG X, et al. Gel polymer electrolyte with ionic liquid for high performance lithium sulfur battery. Solid State Ionics, 2012, 225: 604-607.
DOI URL |
| [16] | GELMAN D, SHVARTSEV B, EIN-ELI Y. Aluminum-air battery based on an ionic liquidelectrolyte. Journal of Physical Chemistry A, 2014, 2: 20237-20242. |
| [17] |
KAZEMIABNAVI S, ZHANG Z, THORNTON K, et al. Electrochemical stability window of imidazolium-based ionic liquids as electrolytes for lithium batteries. Journal of Physical Chemistry B, 2016, 120: 5691-5702.
DOI URL |
| [18] |
LI K, SHAO Y, YAN H, et al. Lattice-contraction triggered synchronous electrochromic actuator. Nature Communications, 2018, 9: 1-11.
DOI URL |
| [19] |
SUN Z, PENG Y, WANG M, et al. Electrochemical deposition of Cu metal-organic framework films for the dual analysis of pathogens. Analytical Chemistry, 2021, 93(25): 8994-9001.
DOI URL |
| [20] | WU F, FANG W, YANG X, et al. Two-dimensional-conjugated metal-organic framework with high electrical conductivity for electrochemical sensing. Journal of the Chinese Society, 2019, 66(5): 522-528. |
| [21] |
LIU J, YANG D, ZHOU Y, et al. Tricycloquinazoline-based 2D conductive metal-organic frameworks as promising electrocatalysts for CO2 reduction. Angewandte Chemie International Edition, 2021, 60(26): 14473-14479.
DOI URL |
| [22] | LI R, LI S, ZHANG Q, et al. Layer-by-layer assembled triphenylene-based MOFs films for electrochromic electrode. Inorganic Chemistry Communications, 2021, 123: 108354. |
| [23] | GUARR T, ANSON C. Electropolymerization of ruthenium (bis (1, 10-phenanthroline) (4-methyl-4’-vinyl2, 2’-bipyridine) complexes through direct attack on the ligand ring system. Journal of Physical Chemistry, 1987, 91: 4037-4043. |
| [24] |
LIANG H, LI R, LI C, et al. Regulation of carbon content in MOF-derived hierarchical-porous NiO@C films for high-performance electrochromism. Materials Horizons, 2019, 6(3): 571-579.
DOI URL |
| [25] |
SONG X, WANG X, LI Y, et al. 2D semiconducting metal-organic framework thin films for organic spin valves. Angewandte Chemie International Edition, 2020, 59(3): 1118-1123.
DOI URL |
| [26] |
NINAWE P, GUPTA K, BALLAV N. Chemically integrating a 2D metal-organic framework with 2D functionalized graphene. Inorganic Chemistry, 2021, 60(24): 19079-19085.
DOI URL |
| [27] |
AMMAR F, SAVEANT J. Convolution potential sweep voltammetry: Part IV. Homogenrous follow-up chemical-reactions. Journal of Electroanalytical Chemistry, 1975, 61: 251-263.
DOI URL |
| [1] | 陈明月, 颜志超, 陈静, 李敏娟, 刘志勇, 蔡传兵. YBa2Cu3O7-δ薄膜的BaCl2/BaF2-MOD法制备及超导特性研究[J]. 无机材料学报, 2023, 38(2): 199-204. |
| [2] | 谢兵, 蔡金峡, 王铜铜, 刘智勇, 姜胜林, 张海波. 高储能密度聚合物基多层复合电介质的研究进展[J]. 无机材料学报, 2023, 38(2): 137-147. |
| [3] | 张家强, 邹馨蕾, 王能泽, 贾春阳. 两步电沉积法制备Zn-Fe PBA薄膜及其在电致变色器件中的性能研究[J]. 无机材料学报, 2022, 37(9): 961-968. |
| [4] | 邓陶丽, 陈河莘, 黑玲丽, 李淑星, 解荣军. 第二相引入荧光转换材料实现激光驱动高均匀性白光光源[J]. 无机材料学报, 2022, 37(8): 891-896. |
| [5] | 程玮杰, 王明磊, 林国强. 电弧离子镀CrAlN-DLC硬质复合薄膜的成分、结构与性能[J]. 无机材料学报, 2022, 37(7): 764-772. |
| [6] | 洪佳辉, 马冉, 仵云超, 文涛, 艾玥洁. MOFs自牺牲模板法制备CoNx/g-C3N4纳米材料用作高效光催化还原U(VI)[J]. 无机材料学报, 2022, 37(7): 741-749. |
| [7] | 黄郅航, 滕官宏伟, 铁鹏, 范德松. 钙钛矿陶瓷薄膜的电致变色特性[J]. 无机材料学报, 2022, 37(6): 611-616. |
| [8] | 赵玉垚, 欧阳俊. 硅片上集成高介电调谐率的柱状纳米晶BaTiO3铁电薄膜[J]. 无机材料学报, 2022, 37(6): 596-602. |
| [9] | 曹志军, 李在均. 钌-生物质碳人工酶的制备及在比色检测杀虫剂毒死蜱残留中的应用[J]. 无机材料学报, 2022, 37(5): 554-560. |
| [10] | 杨柱, 郭少波, 蔡恒辉, 董显林, 王根水. 高分子辅助沉积法制备LaNiO3外延导电薄膜[J]. 无机材料学报, 2022, 37(5): 561-566. |
| [11] | 王万海, 周杰, 唐卫华. 钙钛矿薄膜缺陷调控策略在太阳能电池中的应用[J]. 无机材料学报, 2022, 37(2): 129-139. |
| [12] | 夏求应, 孙硕, 昝峰, 徐璟, 夏晖. 非晶LiSiON薄膜电解质的全固态薄膜锂电池性能[J]. 无机材料学报, 2022, 37(2): 230-236. |
| [13] | 刘丹, 赵亚欣, 郭锐, 刘艳涛, 张志东, 张增星, 薛晨阳. 退火条件对磁控溅射MgO-Ag3Sb-Sb2O4柔性薄膜热电性能的影响[J]. 无机材料学报, 2022, 37(12): 1302-1310. |
| [14] | 王晶, 徐守冬, 卢中华, 赵壮壮, 陈良, 张鼎, 郭春丽. 钠离子电池中空结构CoSe2/C负极材料的制备及储钠性能研究[J]. 无机材料学报, 2022, 37(12): 1344-1350. |
| [15] | 郭隐犇, 陈子曦, 王宏志, 张青红. 基于无机填料复合薄膜的摩擦纳米发电机研究进展[J]. 无机材料学报, 2021, 36(9): 919-928. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||