无机材料学报 ›› 2022, Vol. 37 ›› Issue (8): 873-882.DOI: 10.15541/jim20210798
胡越1( ), 安琳1, 韩鑫2(
), 安琳1, 韩鑫2( ), 侯成义1, 王宏志1, 李耀刚3, 张青红3(
), 侯成义1, 王宏志1, 李耀刚3, 张青红3( )
)
收稿日期:2022-12-29
修回日期:2022-03-09
出版日期:2022-08-20
网络出版日期:2022-03-10
通讯作者:
张青红, 教授. E-mail: zhangqh@dhu.edu.cn;作者简介:胡 越(1998-), 女, 硕士研究生. E-mail: yue_hamish@163.com
基金资助:
HU Yue1( ), AN Lin1, HAN Xin2(
), AN Lin1, HAN Xin2( ), HOU Chengyi1, WANG Hongzhi1, LI Yaogang3, ZHANG Qinghong3(
), HOU Chengyi1, WANG Hongzhi1, LI Yaogang3, ZHANG Qinghong3( )
)
Received:2022-12-29
Revised:2022-03-09
Published:2022-08-20
Online:2022-03-10
Contact:
ZHANG Qinghong, professor. E-mail: zhangqh@dhu.edu.cn;About author:HU Yue (1998-), female, Master candidate. E-mail: yue_hamish@163.com
Supported by:摘要:
钒酸铋是最具有光电催化应用潜力的水分解光电阳极之一, 但由于表面缓慢的动力学反应速率, 其光电催化效率仍不理想。本研究通过浸渍法在BiVO4薄膜光阳极上负载纳米RhO2助催化剂, 研究RhO2负载量对BiVO4光阳极光电催化性能的影响规律及其机理。晶粒尺寸10~25 nm的RhO2均匀负载在颗粒尺寸100~250 nm、厚度约为400 nm的BiVO4光阳极薄膜表面。考虑到贵金属铑的昂贵成本, RhO2的最佳负载量为质量分数1.65%, 在偏压1.23 V (vs. RHE)、1.0 mol/L Na2SO3溶液中(pH8.5)AM 1.5模拟可见光照射下, 光电流密度达3.81 mA·cm-2, 相较纯BiVO4提升了10.58倍。在没有有机牺牲剂的条件下, 光阳极同时析出了氢气和氧气, 两者比例接近2 : 1, 产氧速率为8.22 μmol/(h·cm2)。负载RhO2有效改善了光阳极的表面水氧化动力学, 使光生空穴更快与电解质溶液进行水氧化反应, 抑制光生载流子复合, 从而显著提升光电催化性能。另外, 负载RhO2后, 空穴更容易从光阳极表面被有效提取到电解质溶液中, 减少其在光阳极表面积累, 从而使BiVO4/RhO2(1.65%)光阳极可持续稳定工作10 h以上。
中图分类号:
胡越, 安琳, 韩鑫, 侯成义, 王宏志, 李耀刚, 张青红. RhO2修饰BiVO4薄膜光阳极的制备及其光电催化分解水性能[J]. 无机材料学报, 2022, 37(8): 873-882.
HU Yue, AN Lin, HAN Xin, HOU Chengyi, WANG Hongzhi, LI Yaogang, ZHANG Qinghong. RhO2 Modified BiVO4 Thin Film Photoanodes: Preparation and Photoelectrocatalytic Water Splitting Performance[J]. Journal of Inorganic Materials, 2022, 37(8): 873-882.
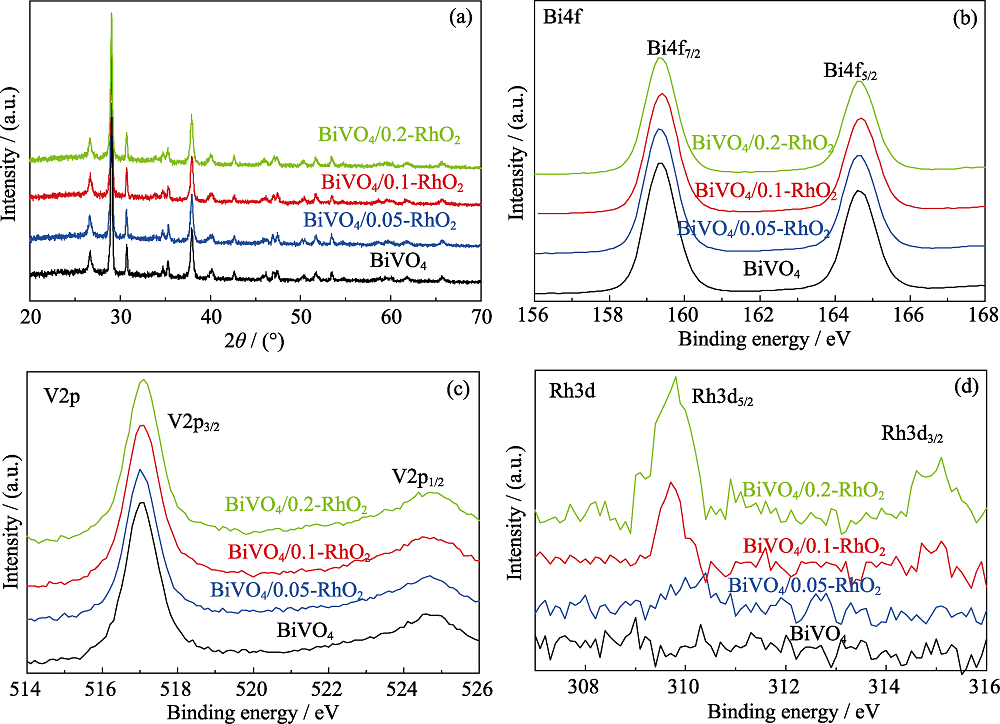
图1 不同负载量RhO2修饰的BiVO4光阳极的(a)XRD图谱, 以及(b)Bi4f, (c)V2p 和(d)Rh3d的高分辨率XPS图谱
Fig. 1 (a) XRD patterns and XPS spectra of (b) Bi4f, (c) V2p, (d) Rh3d of all BiVO4/RhO2 photoanodes
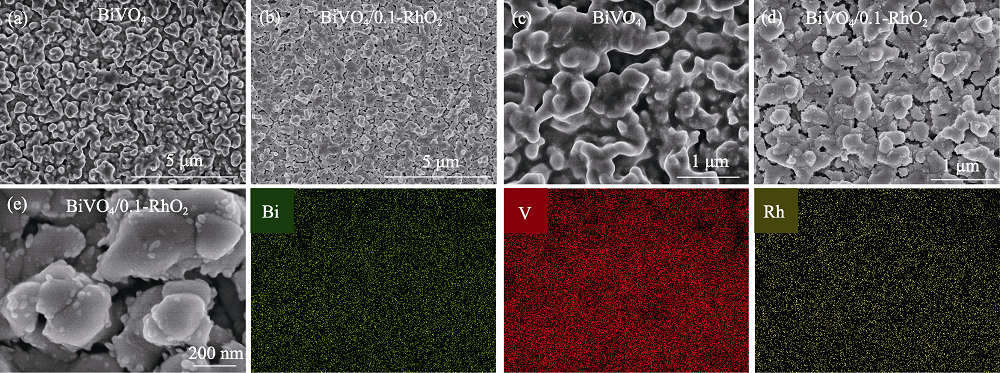
图2 纯BiVO4和BiVO4/0.1-RhO2的(a, b)低倍和(c, d)高倍场发射扫描电镜照片, (e) BiVO4/0.1-RhO2高倍场发射扫描电镜照片及其Bi、V、Rh的元素分布图
Fig. 2 (a, b) Low-magnification and (c, d) high-magnification FESEM images of bare BiVO4 surface and BiVO4/0.1-RhO2 photoanodes, and (e) high-magnification SEM image with elemental mappings of the BiVO4/0.1-RhO2 photoanode
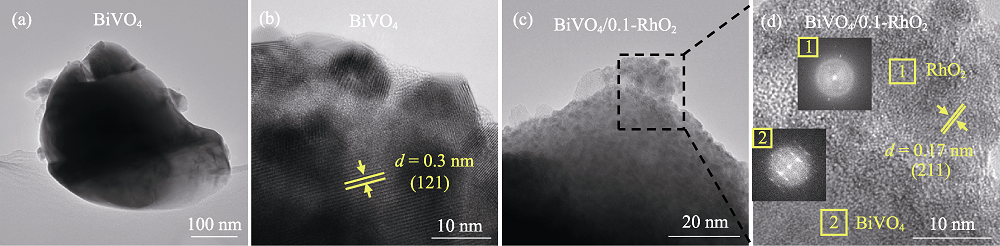
图3 (a, b)纯BiVO4和(c, d) BiVO4/0.1-RhO2的(a, c) TEM和(b, d) HRTEM照片
Fig. 3 (a, c) TEM and (b, d) HRTEM images of (a, b) bare BiVO4 and (c, d) BiVO4/0.1-RhO2 photoanodes The inset in (d) is the SAED patterns of the selected area
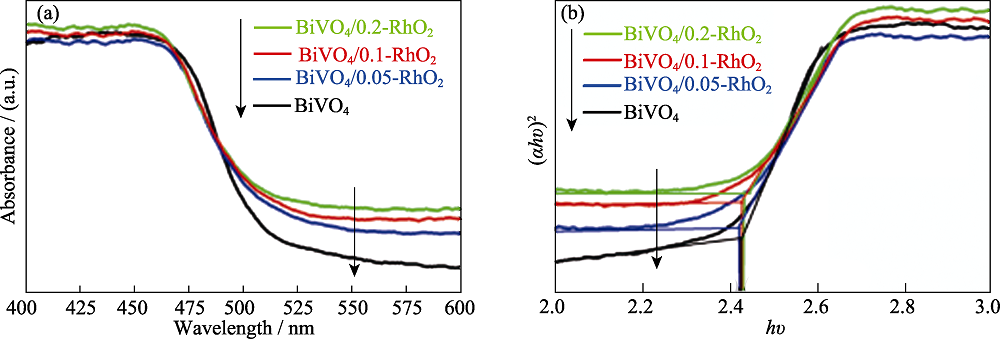
图4 不同负载量RhO2修饰的BiVO4光阳极的(a) UV-Vis DRS光谱和(b) (αhν) 2 vs hν的tauc图
Fig. 4 (a) UV-Vis DRS spectra and (b) (αhν) 2 vs hν tauc plots of BiVO4/RhO2 photoanodes
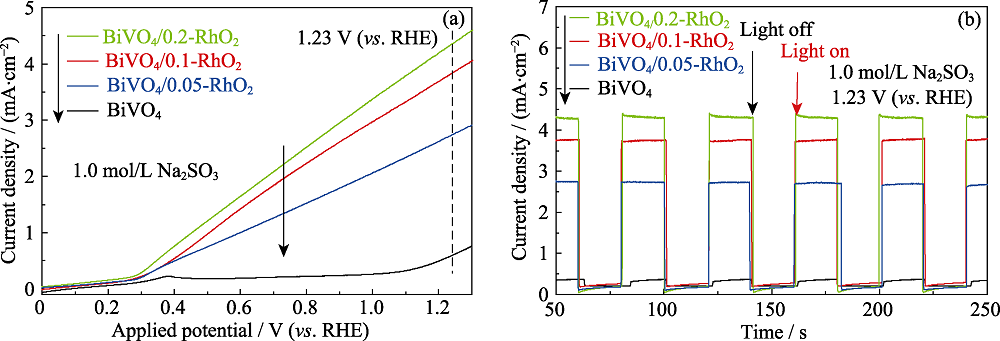
图5 不同负载量RhO2修饰的BiVO4光阳极的(a)线性扫描伏安(LSV)曲线和(b)光电流响应曲线, 测试电解液为1.0 mol/L Na2SO3 (pH8.5)
Fig. 5 (a) Liner sweep voltammetry curves and (b) photocurrent response plots of BiVO4/RhO2 photoanodes in 1.0 mol/L Na2SO3 (pH8.5) electrolyte
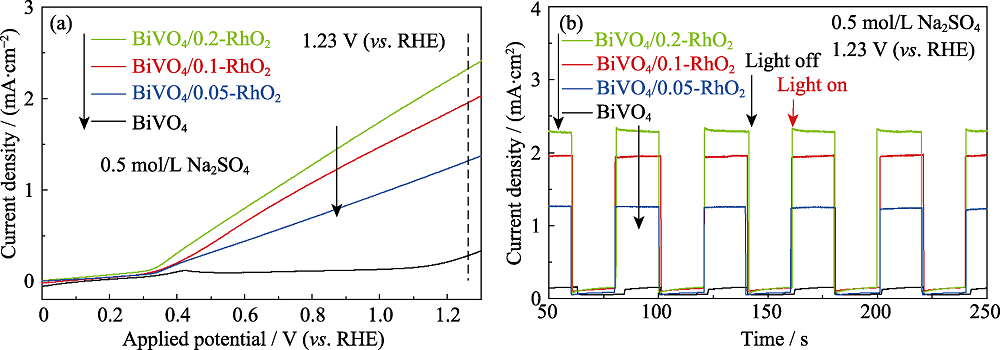
图6 不同负载量RhO2修饰的BiVO4光阳极的(a)线性扫描伏安(LSV)曲线和(b)光电流响应曲线, 测试电解液为0.5 mol/L Na2SO4 (pH6.8)
Fig. 6 (a) Liner sweep voltammetry curves and (b) photocurrent response plots of all BiVO4/RhO2 photoanodes in 0.5 mol/L Na2SO4 (pH6.8) electrolyte
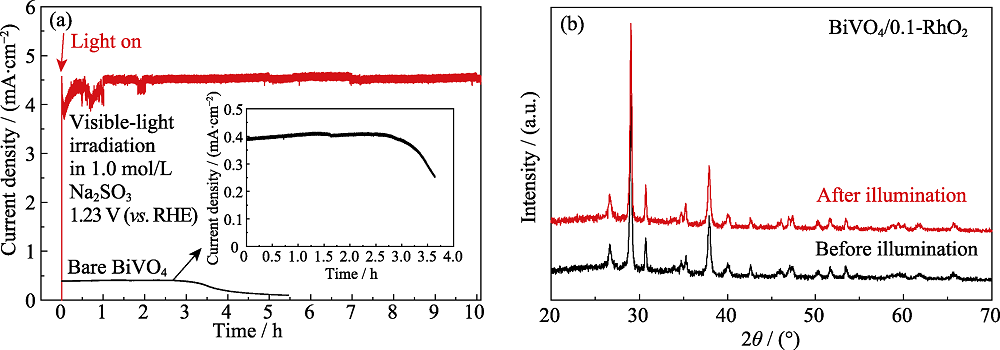
图8 可见光照射、外加偏压为1.23 V (vs. RHE)、电解液为1.0 mol/L Na2SO3 (pH8.5)条件下BiVO4/0.1-RhO2光阳极的(a)光电流稳定性和(b)催化前后的XRD图谱
Fig. 8 (a) Photocurrent stability of the BiVO4/0.1-RhO2 photoanode in 1.0 mol/L Na2SO3 (pH8.5) under visible-light illumination, and (b) XRD patterns of the BiVO4/0.1-RhO2 photoanodes before and after illumination, respectively
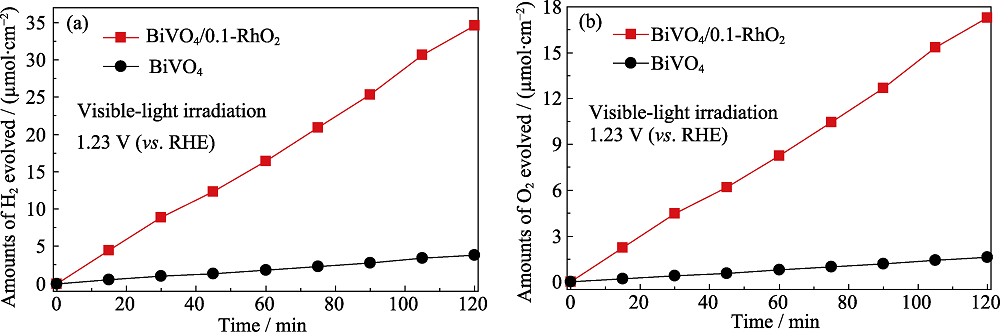
图9 在可见光激发、外加偏压为1.23 V (vs. RE)、电解液为1.0 mol/L Na2SO3 (pH8.5)条件下BiVO4/0.1-RhO2光阳极的(a)产氢速率曲线和(b)产氧速率曲线
Fig. 9 (a) Hydrogen and (b) oxygen evolution vs. reaction time per illuminated area for bare BiVO4 and BiVO4/RhO2 photoanodes under visible-light irradiation with the electrolyte of 1.0 mol/L Na2SO3 (pH8.5) as hole scavenger
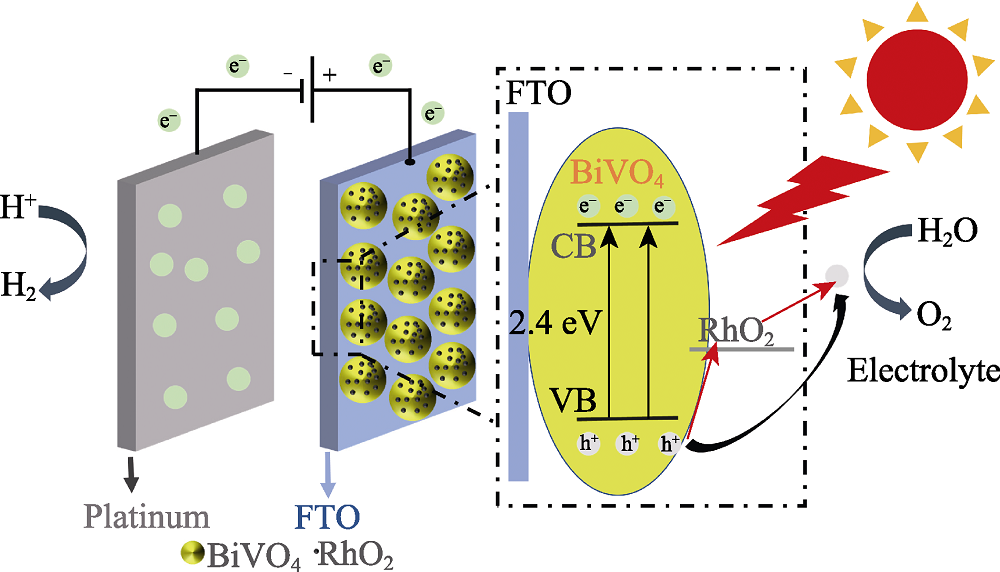
图10 RhO2修饰的BiVO4光阳极在可见光照射下进行光电催化分解水的机理示意图
Fig. 10 Schematic diagram depicting PEC water splitting of the RhO2-modified BiVO4 photoanode under visible light irradiation
| [1] | FANG M, CAI Q, QIN Q, et al. Mo-doping induced crystal orientation reconstruction and oxygen vacancy on BiVO4 homojunction for enhanced solar-driven water splitting. Chemical Engineering Journal, 2021, 421: 127796. |
| [2] |
ZHANG W, SHEN Q, XUE J, et al. Preparation and photoelectrochemical water oxidation of hematite nanobelts containing highly ordered oxygen vacancies. Journal of Inorganic Materials, 2021, 36(12): 1290-1296.
DOI URL |
| [3] | HAN X, SI T, LIU Q, et al. 2D bimetallic RuNi alloy co-catalysts remarkably enhanced the photocatalytic H2 evolution performance of g-C3N4 nanosheets. Chemical Engineering Journal, 2021, 426: 130824. |
| [4] | SU K, ZHANG Y, LU F, et al. Platinum decorated titanium dioxide nanosheets for efficient photoelectrocatalytic hydrogen evolution reaction. Journal of Inorganic Materials, 2019, 34(11): 1200-1204. |
| [5] |
KIM J H, LEE J S. Elaborately modified BiVO4 photoanodes for solar water splitting. Advanced Materials, 2019, 31(20): 1806938.
DOI URL |
| [6] |
HE Y M, HAMANN T, WANG D W. Thin film photoelectrodes for solar water splitting. Chemical Society Reviews, 2019, 48(7): 2182-2215.
DOI URL |
| [7] |
BAE D, SEGER B, VESBORG P C K, et al. Strategies for stable water splitting via protected photoelectrodes. Chemical Society Reviews, 2017, 46(7): 1933-1954.
DOI URL |
| [8] | LU X, YE K H, ZHANG S, et al. Amorphous type FeOOH modified defective BiVO4 photoanodes for photoelectrochemical water oxidation. Chemical Engineering Journal, 2022, 428: 131027. |
| [9] |
JIAN J, JIANG G S, VAN DE KROL R, et al. Recent advances in rational engineering of multinary semiconductors for photoelectrochemical hydrogen generation. Nano Energy, 2018, 51: 457-480.
DOI URL |
| [10] |
GAO Y, FAN W, QU K, et al. Confined growth of Co-Pi co- catalyst by organic semiconductor polymer for boosting the photoelectrochemical performance of BiVO4. New Journal of Chemistry, 2019, 43(21): 8160-8167.
DOI URL |
| [11] |
PENG B, XIA M, LI C, et al. Network structured CuWO4/BiVO4/ Co-Pi nanocomposite for solar water splitting. Catalysts, 2018, 8(12): 663-672.
DOI URL |
| [12] | YE K H, WANG Z L, GU J W, et al. Carbon quantum dots as a visible light sensitizer to significantly increase the solar water splitting performance of bismuth vanadate photoanodes. Energy & Environmental Science, 2017, 10(3): 772-779. |
| [13] |
ZENG Q Y, LI J H, LI L S, et al. Synthesis of WO3/BiVO4 photoanode using a reaction of bismuth nitrate with peroxovanadate on WO3 film for efficient photoelectrocatalytic water splitting and organic pollutant degradation. Applied Catalysis B-Environmental, 2017, 217: 21-29.
DOI URL |
| [14] |
ZHONG D K, CHOI S, GAMELIN D R. Near-complete suppression of surface recombination in solar photoelectrolysis by "Co-Pi" catalyst-modified W: BiVO4. Journal of the American Chemical Society, 2011, 133(45): 18370-18377.
DOI URL |
| [15] |
RITLENG V, SIRLIN C, PFEFFER M. Ru-, Rh-, and Pd-catalyzed C-C bond formation involving C-H activation and addition on unsaturated substrates: reactions and mechanistic aspects. Chemical Reviews, 2002, 102(5): 1731-1769.
DOI URL |
| [16] |
SONG G, WANG F, LI X. C-C, C-O and C-N bond formation via rhodium (III)-catalyzed oxidative C-H activation. Chemical Society Reviews, 2012, 41(9): 3651-3678.
DOI URL |
| [17] |
CAMPBELL C T. Ultrathin metal films and particles on oxide surfaces: structural, electronic and chemisorptive properties. Surface Science Reports, 1997, 27(1/2/3): 1-111.
DOI URL |
| [18] |
COLBY D A, TSAI A S, BERGMAN R G, et al. Rhodium catalyzed chelation-assisted C-H bond functionalization reactions. Accounts of Chemical Research, 2012, 45(6): 814-825.
DOI URL |
| [19] |
OHNO T, BAI L, HISATOMI T, et al. Photocatalytic water splitting using modified GaN:ZnO solid solution under visible light: long-time operation and regeneration of activity. Journal of the American Chemical Society, 2012, 134(19): 8254-8259.
DOI URL |
| [20] |
WANG S, CHEN P, YUN J H, et al. An electrochemically treated BiVO4 photoanode for efficient photoelectrochemical water splitting. Angewandte Chemie International Edition, 2017, 56(29): 8500-8504.
DOI URL |
| [21] | MIAO Y, LIU J, CHEN L, et al. Single-atomic-Co cocatalyst on (040) facet of BiVO4 toward efficient photoelectrochemical water splitting. Chemical Engineering Journal, 2022, 427: 131011. |
| [22] |
PERRY S C, PANGOTRA D, VIEIRA L, et al. Electrochemical synthesis of hydrogen peroxide from water and oxygen. Nature Reviews Chemistry, 2019, 3(7): 442-458.
DOI URL |
| [23] |
ZHANG Y P, LI Y, NI D Q, et al. Improvement of BiVO4 photoanode performance during water photo-oxidation using Rh-doped SrTiO3 perovskite as a co-catalyst. Advanced Functional Materials, 2019, 29(32): 1902101.
DOI URL |
| [24] | FEI H, SHAO J, LI H, et al. Construction of ultra-thin 2D CN-Br- 0.12/2% RhOx photo-catalyst with rapid electron and hole separation for efficient bisphenol A degradation. Applied Catalysis B- Environmental, 2021, 299: 120623. |
| [25] |
LEITE E R, MACIEL A P, WEBER I T, et al. Development of metal oxide nanoparticles with high stability against particle growth using a metastable solid solution. Advanced Materials, 2002, 14(12): 905-908.
DOI URL |
| [26] |
FOUNTAINE K T, LEWERENZ H J, ATWATER H A. Interplay of light transmission and catalytic exchange current in photoelectrochemical systems. Applied Physics Letters, 2014, 105(17): 173901.
DOI URL |
| [27] |
TOLOD K R, HERNANDEZ S, RUSSO N. Recent advances in the BiVO4 photocatalyst for sun-driven water oxidation: top-performing photoanodes and scale-up challenges. Catalysts, 2017, 7(1): 13-23.
DOI URL |
| [28] | SHE H, YUE P, HUANG J, et al. One-step hydrothermal deposition of F:FeOOH onto BiVO4 photoanode for enhanced water oxidation. Chemical Engineering Journal, 2020, 392: 123703. |
| [29] | JU S, JUN J, HUH D, et al. Simultaneous improvement of absorption and separation efficiencies of Mo:BiVO4 photoanodes via nanopatterned SnO2/Au hybrid layers. ACS Sustainable Chemistry & Engineering, 2019, 7(20): 17000-17007. |
| [30] |
QI J, KONG D, LIU D, et al. Bimetallic phosphide decorated Mo-BiVO4 for significantly improved photoelectrochemical activity and stability. RSC Advances, 2019, 9(27): 15629-15634.
DOI URL |
| [31] | YIN X, QIU W, LI W, et al. High porosity Mo doped BiVO4 film by vanadium re-substitution for efficient photoelectrochemical water splitting. Chemical Engineering Journal, 2020, 389: 124365. |
| [32] |
JIAN J, XU Y, YANG X, et al. Embedding laser generated nanocrystals in BiVO4 photoanode for efficient photoelectrochemical water splitting. Nature Communications, 2019, 10(1): 2609.
DOI URL |
| [33] |
COSTANTINO F, KAMAT P V. Do sacrificial donors donate H2 in photocatalysis? ACS Energy Letters, 2021, 7: 242-246.
DOI URL |
| [34] |
MAEDA K, XIONG A, YOSHINAGA T, et al. Photocatalytic overall water splitting promoted by two different cocatalysts for hydrogen and oxygen evolution under visible light. Angewandte Chemie International Edition, 2010, 49(24): 4096-4099.
DOI URL |
| [35] |
WANG D, LI R, ZHU J, et al. Photocatalytic water oxidation on BiVO4 with the electrocatalyst as an oxidation cocatalyst: essential relations between electrocatalyst and photocatalyst. The Journal of Physical Chemistry C, 2012, 116(8): 5082-5089.
DOI URL |
| [1] | 王如意, 徐国良, 杨蕾, 邓崇海, 储德林, 张苗, 孙兆奇. p-n异质结BiVO4/g-C3N4光阳极的制备及其光电化学水解性能[J]. 无机材料学报, 2023, 38(1): 87-96. |
| [2] | 陈瀚翔, 周敏, 莫曌, 宜坚坚, 李华明, 许晖. CoN/g-C3N4 0D/2D复合结构及其光催化制氢性能研究[J]. 无机材料学报, 2022, 37(9): 1001-1008. |
| [3] | 安琳, 吴淏, 韩鑫, 李耀刚, 王宏志, 张青红. 非贵金属Co5.47N/N-rGO助催化剂增强TiO2光催化制氢性能[J]. 无机材料学报, 2022, 37(5): 534-540. |
| [4] | 高娃, 熊宇杰, 吴聪萍, 周勇, 邹志刚. 基于超薄纳米结构的光催化二氧化碳选择性转化[J]. 无机材料学报, 2022, 37(1): 3-14. |
| [5] | 张文进, 申倩倩, 薛晋波, 李琦, 刘旭光, 贾虎生. 具有高度有序氧空位的α-Fe2O3纳米带的制备及光电催化水氧化性能研究[J]. 无机材料学报, 2021, 36(12): 1290-1296. |
| [6] | 徐晶威,李政,王泽普,于涵,何祺,付念,丁帮福,郑树凯,闫小兵. 交错能带结构钕掺杂钒酸铋形貌与光催化性能调控[J]. 无机材料学报, 2020, 35(7): 789-795. |
| [7] | 张翊青,张淑娟,万正睿,莫晗,王念贵,周立群. RuFe纳米粒子修饰片状BiVO4协同催化氨硼烷水解产氢[J]. 无机材料学报, 2020, 35(7): 809-816. |
| [8] | 郑云,陈亦琳,高碧芬,林碧洲. 磷烯光催化分解水研究进展[J]. 无机材料学报, 2020, 35(6): 647-653. |
| [9] | 苏琨, 张亚茹, 陆飞, 张君, 王熙. 铂修饰二氧化钛纳米片的制备及其光电催化析氢反应研究[J]. 无机材料学报, 2019, 34(11): 1200-1204. |
| [10] | 王松灿, 汤枫秋, 王连洲. 光电催化分解水用可见光响应型氧化物光阳极的改性研究进展[J]. 无机材料学报, 2018, 33(2): 173-197. |
| [11] | 余洁意, 黄 昊, 高 见, 周 雷, 高 嵩, 董星龙, 全 燮. 直流电弧等离子体制备纳米SiC及其催化特性[J]. 无机材料学报, 2017, 32(4): 351-356. |
| [12] | 郭冬雪, 张青红, 王宏志, 李耀刚, 曹广秀. RuO2/ZrO2/TaON复合光催化材料的制备及光解水制氢性能[J]. 无机材料学报, 2015, 30(10): 1025-1030. |
| [13] | 王 敏, 刘 琼, 孙亚杰, 车寅生, 姜承志. 溶胶-凝胶法制备Eu3+掺杂BiVO4及其可见光光催化性能[J]. 无机材料学报, 2013, 28(2): 153-158. |
| [14] | 郭 佳, 朱 毅, 张渊明, 李明玉, 杨 骏. 不同结构形貌BiVO4的水热制备及可见光催化性能[J]. 无机材料学报, 2012, 27(1): 26-32. |
| [15] | 申延明, 刘 丹, 吴 静, 刘雅祺, 姬生菲, 李天舒. 由蜂窝状结构TBT/PMMA杂化膜制备有序孔结构的TiO2膜[J]. 无机材料学报, 2010, 25(5): 485-489. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||