无机材料学报 ›› 2022, Vol. 37 ›› Issue (11): 1225-1235.DOI: 10.15541/jim20220040
所属专题: 【生物材料】骨骼与齿类组织修复
收稿日期:2022-01-21
修回日期:2022-02-18
出版日期:2022-03-10
网络出版日期:2022-03-10
通讯作者:
朱敏, 副教授. E-mail: mzhu@usst.edu.cn;作者简介:舒朝琴(1995-), 女, 硕士研究生. E-mail: 1573738940@qq.com
基金资助:
SHU Chaoqin1,2( ), ZHU Min1(
), ZHU Min1( ), ZHU Yufang2(
), ZHU Yufang2( )
)
Received:2022-01-21
Revised:2022-02-18
Published:2022-03-10
Online:2022-03-10
Contact:
ZHU Min, associate professor. E-mail: mzhu@usst.edu.cn;About author:SHU Chaoqin (1995-), female, Master candidate. E-mail: 1573738940@qq.com
Supported by:摘要:
生物活性陶瓷骨修复材料虽然具有优异的成骨性能, 但缺乏抗氧化应激的能力, 妨碍骨修复进程。本研究以β相磷酸三钙(β-TCP)粉体为原料, 采用LiCl-KCl熔盐体系, 以六水合氯化钴(CoCl2·6H2O)为钴源, 利用熔盐法制备出含钴氯磷灰石(Co-MS-TCP)。通过Co-MS-TCP粉体清除过氧化氢(H2O2)分析了含钴氯磷灰石的抗氧化能力; 通过细胞活性、胞内活性氧(ROS)含量变化评价了材料的细胞相容性和细胞水平抗氧化性能。结果表明, 熔盐处理β-TCP粉体能够制备含钴氯磷灰石, 钴含量随CoCl2·6H2O加入量增加而增大; H2O2清除能力随氯磷灰石中钴含量的增加而增强, 6 h内对H2O2的清除率可达90%以上。细胞实验证实, 含钴氯磷灰石具有良好的细胞相容性和抗氧化性能, 1.5 mg·mL-1加3% Co盐的MS-TCP (3%Co-MS-TCP)即可保证软骨细胞和骨髓间充质干细胞存活率大于85%, 并且3% Co-MS-TCP可有效清除H2O2, 使得细胞内ROS含量显著降低。因此, 通过熔盐法制备含钴生物活性陶瓷是实现抗氧化应激的一种有效途径, 这也为开发催化活性高、生物相容好的功能化生物活性陶瓷提供了新的策略。
中图分类号:
舒朝琴, 朱敏, 朱钰方. 熔盐法制备含钴氯磷灰石及其抗氧化性能和细胞相容性研究[J]. 无机材料学报, 2022, 37(11): 1225-1235.
SHU Chaoqin, ZHU Min, ZHU Yufang. Cobalt-incorporated Chlorapatite: Preparation by Molten Salt Method, Anti-oxidation and Cytocompatibility[J]. Journal of Inorganic Materials, 2022, 37(11): 1225-1235.
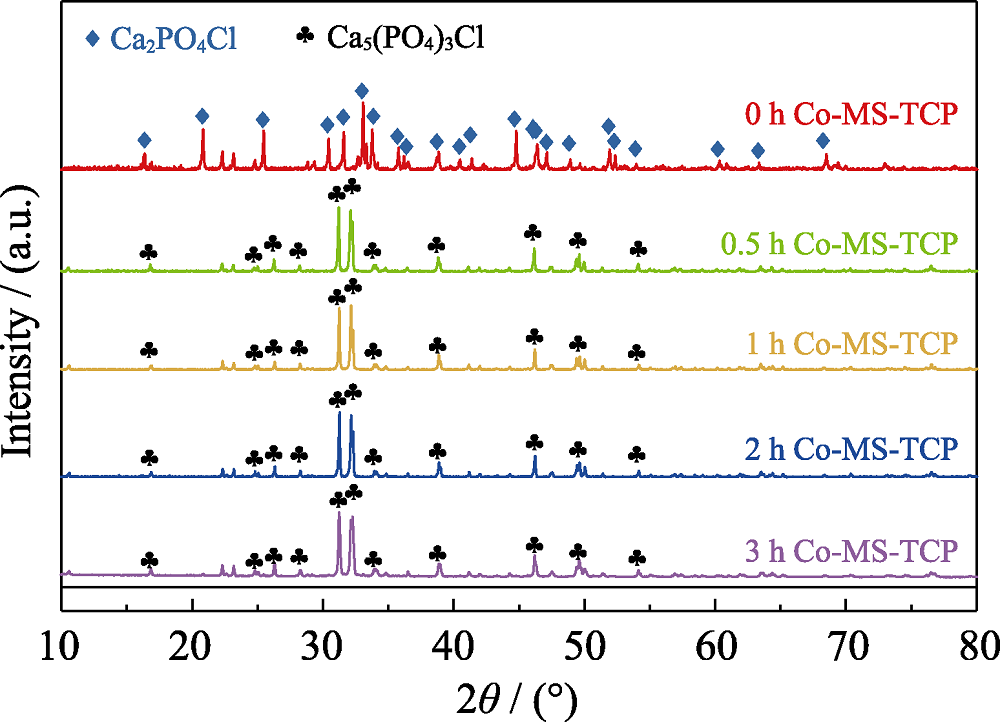
图1 熔盐处理保温不同时间制备的Co-MS-TCP粉体的XRD图谱
Fig. 1 XRD patterns of Co-MS-TCP powders prepared by molten salt method with different holding time The color figure can be obtained from online edition
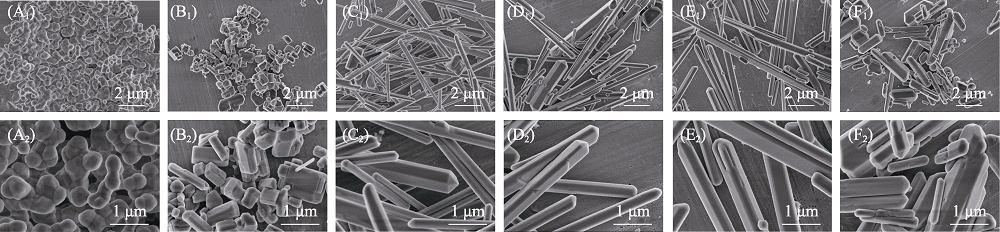
图2 β-TCP粉体经熔盐处理保温不同时间前后样品的微观形貌SEM照片
Fig. 2 SEM images of β-TCP powders before and after molten salt treatment with different holding time (A1-A2) β-TCP; (B1-B2) 0 h Co-MS-TCP; (C1-C2) 0.5 h Co-MS-TCP; (D1-D2) 1 h Co-MS-TCP; (E1-E2) 2 h Co-MS-TCP; (F1-F2) 3 h Co-MS-TCP
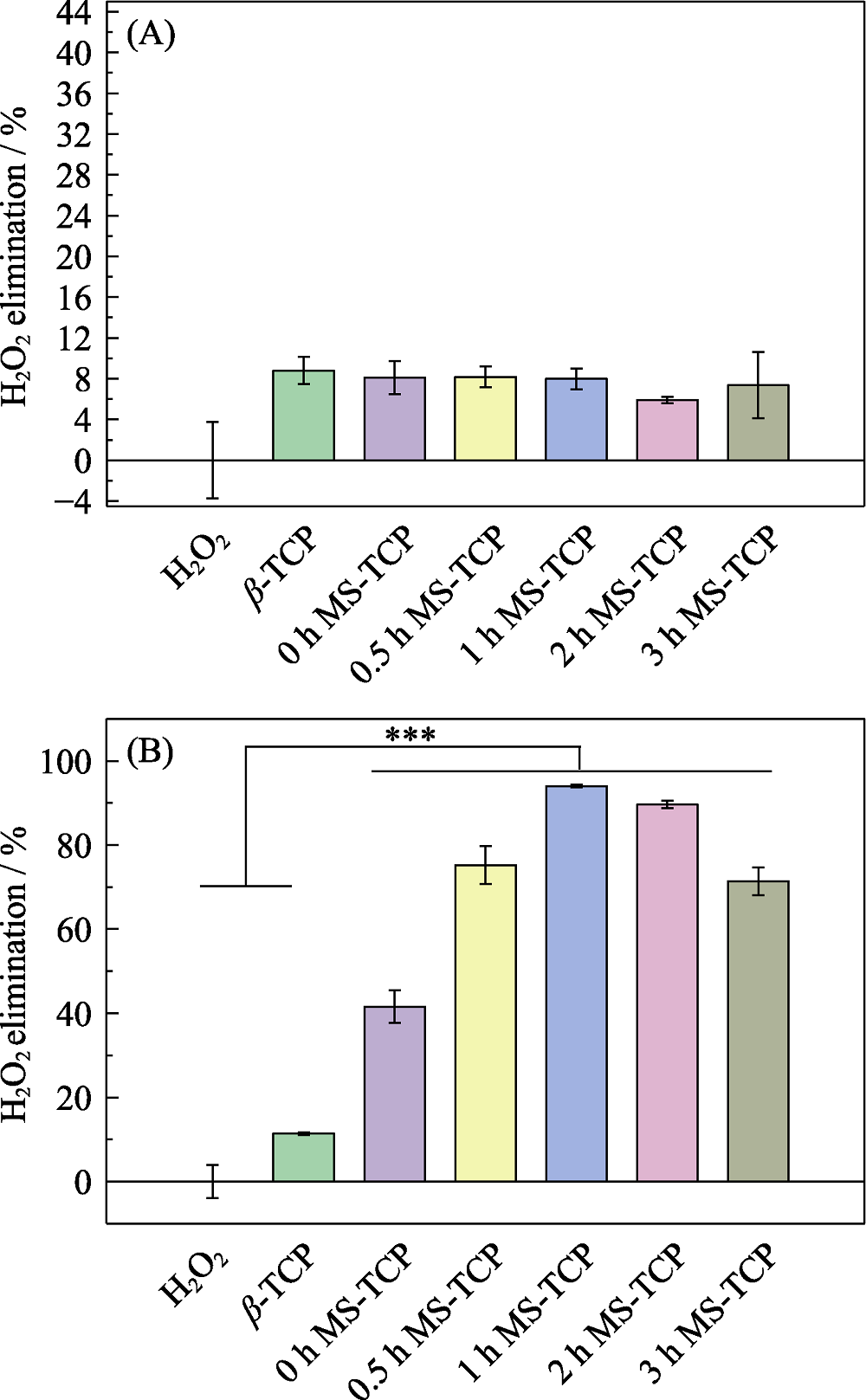
图3 熔盐处理保温不同时间制备的(A)MS-TCP和(B) Co-MS-TCP粉体的清除H2O2情况
Fig. 3 H2O2 scavenging effect of (A) MS-TCP and (B) Co-MS-TCP powders prepared by molten salt method with different holding time *** p<0.001
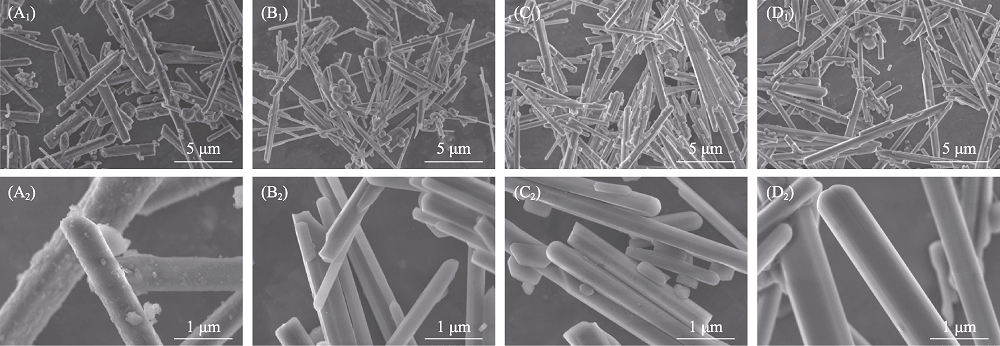
图5 不同钴盐加入量所获熔盐处理制备的Co-MS-TCP粉体的SEM照片
Fig. 5 SEM images of Co-MS-TCP powders prepared by molten salt method with different cobalt salt additions (A1, A2) MS-TCP; (B1, B2) 1% Co-MS-TCP; (C1, C2) 3% Co-MS-TCP; (D1, D2) 5% Co-MS-TCP
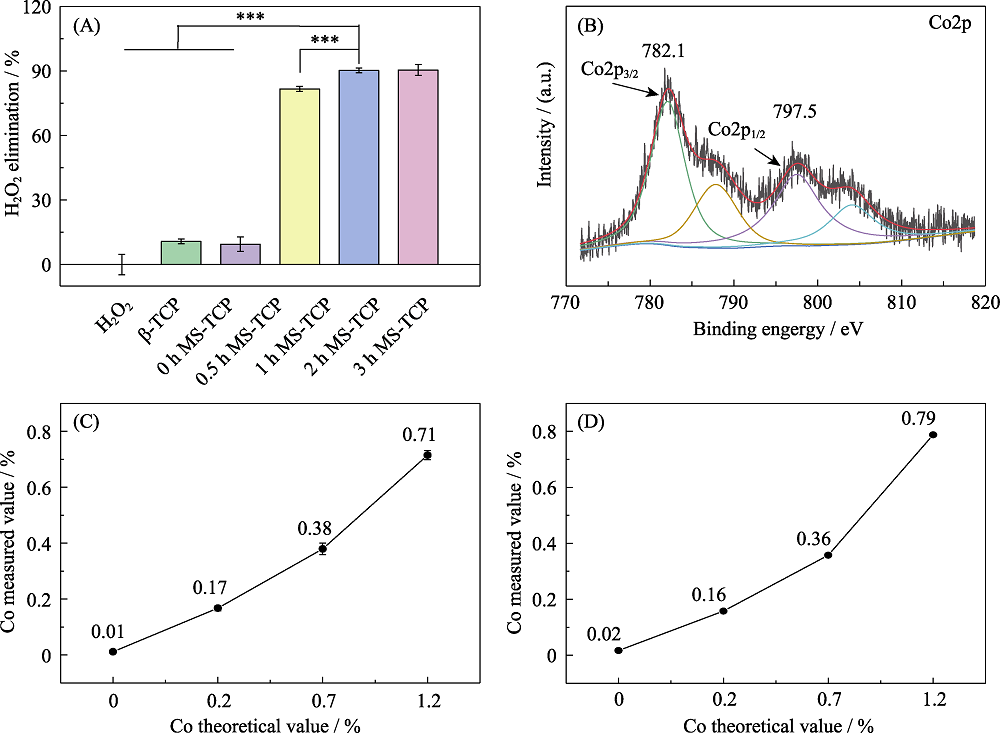
图6 钴含量及其价态在含钴氯磷灰石催化清除H2O2中的作用
Fig. 6 Contents and valence state of cobalt in Co-MS-TCP on H2O2 scavenging ability (A) H2O2 scavenging effect of Co-MS-TCP powders with different cobalt contents; (B) XPS spectra of cobalt in 3% Co-MS-TCP powder; (C, D) Cobalt contents in MS-TCP, 1% Co-MS-TCP, 3% Co-MS-TCP, and 5% Co-MS-TCP powders determined by (C) ICP-AES and (D) XRF methods. *p<0.05, **p<0.01, ***p<0.001. The color figures can be obtained from online edition
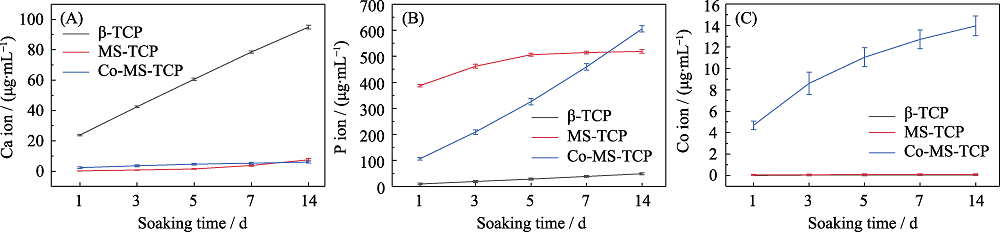
图7 β-TCP、MS-TCP和3%Co-MS-TCP粉体在Tris-HCl缓冲液中(A) Ca、(B) P、(C) Co离子的释放情况
Fig. 7 Releases of (A) Ca, (B) P and (C) Co ions from β-TCP, MS-TCP and 3% Co-MS-TCP powders in Tris-HCl buffer The color figures can be obtained from online edition

图8 SBF溶液中浸泡7 d后的β-TCP (A1, A2)、MS-TCP (B1, B2)和3% Co-MS-TCP (C1, C2)粉体的SEM照片和(D)红外光谱图
Fig. 8 SEM images of (A1, A2) β-TCP, (B1, B2) MS-TCP and (C1, C2) 3% Co-MS-TCP after soaking in SBF for 7 d and (D) their corresponding FT-IR spectra The color figure can be obtained from online edition
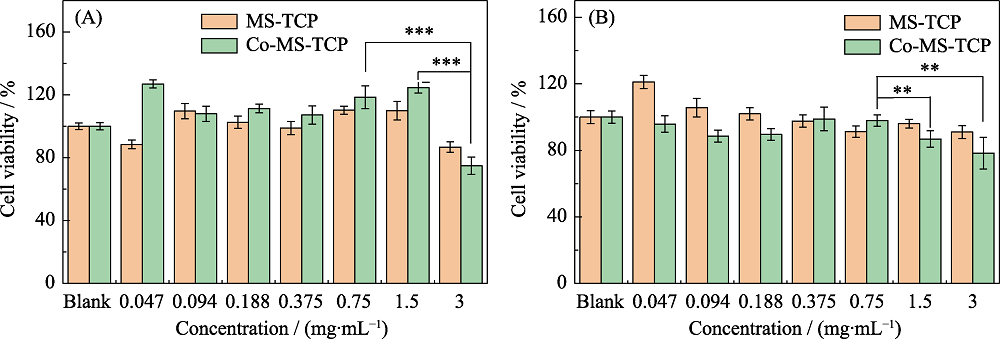
图9 不同浓度MS-TCP和3% Co-MS-TCP 粉体对(A)软骨细胞和(B)骨髓间充质干细胞的细胞毒性评估
Fig. 9 Cell viabilities of MS-TCP and 3% Co-MS-TCP powders with different concentrations on (A) cartilage cells and (B) bone marrow mesenchymal stem cells **p<0.01, *** p<0.001; The color figures can be obtained from online edition
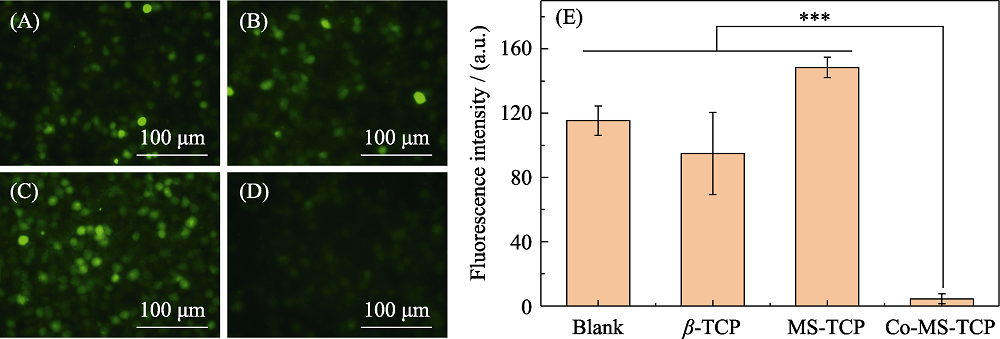
图10 (A)空白组和(B) β-TCP, (C) MS-TCP, (D) 3% Co-MS-TCP粉体处理H2O2刺激的软骨细胞内ROS染色荧光显微镜照片和(E)对应的荧光强度统计
Fig. 10 Images of intracellular ROS staining fluorescence in cartilage cells stimulated by H2O2 treatment with powders (A) Blank, (B) β-TCP, (C) MS-TCP, (D) 3% Co-MS-TCP and (E) corresponding fluorescence intensity statistics ***p<0.001; The color figures can be obtained from online edition
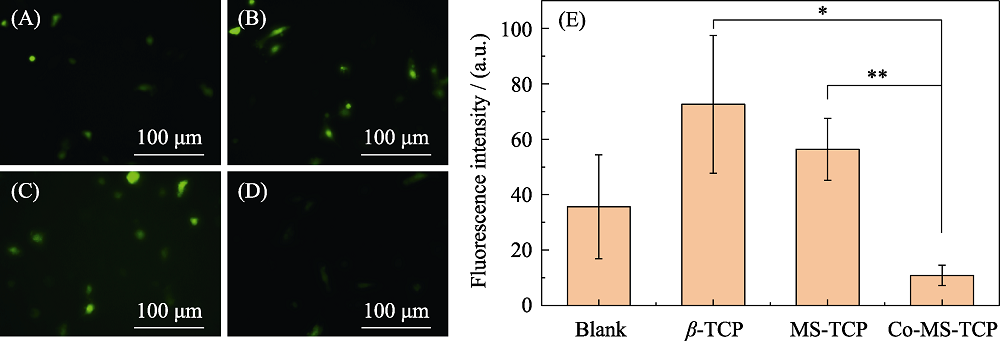
图11 (A)空白组和(B) β-TCP, (C) MS-TCP, (D) 3% Co-MS-TCP粉体处理H2O2刺激的骨髓间充质干细胞后的细胞内ROS染色荧光显微镜照片和(E)对应的荧光强度统计
Fig. 11 Images of intracellular ROS staining fluorescence in bone marrow mesenchymal stem cells stimulated by H2O2 treatment with powders (A) Blank, (B) β-TCP, (C) MS-TCP, (D) 3% Co-MS-TCP and (E) Corresponding fluorescence intensity statistics. * p<0.05, ** p<0.01 The color figures can be obtained from online edition
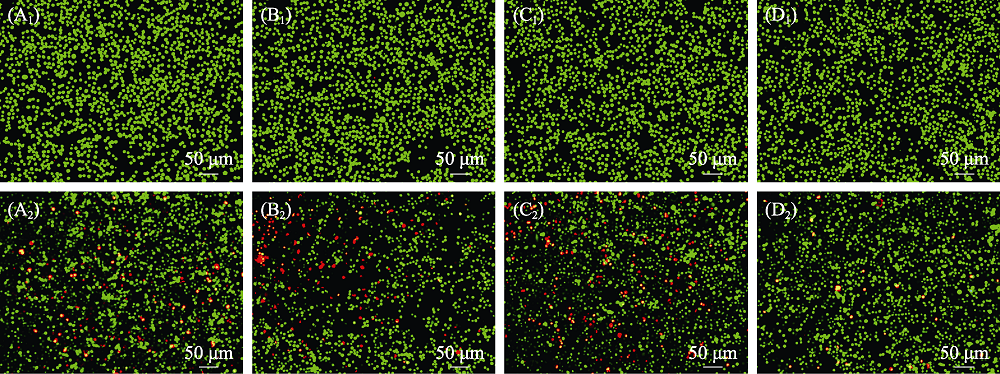
图12 (A)空白组和(B) β-TCP, (C) MS-TCP, (D) 3% Co-MS-TCP粉体处理(A1-D1) 无H2O2刺激与(A2-D2) H2O2刺激的软骨细胞后的细胞活(绿色)/死(红色)Calcein-AM-PI染色荧光显微镜照片
Fig. 12 Calcein AM and PI fluorescence images of cartilage cells treated with different powders((A) Blank; (B) β-TCP; (C) MS-TCP; (D) 3% Co-MS-TCP; (A1-D1) Cells without H2O2 stimulation; (A2-D2) Cells stimulated with H2O2 showing live (green) and death (red) cells) The color figures can be obtained from online edition
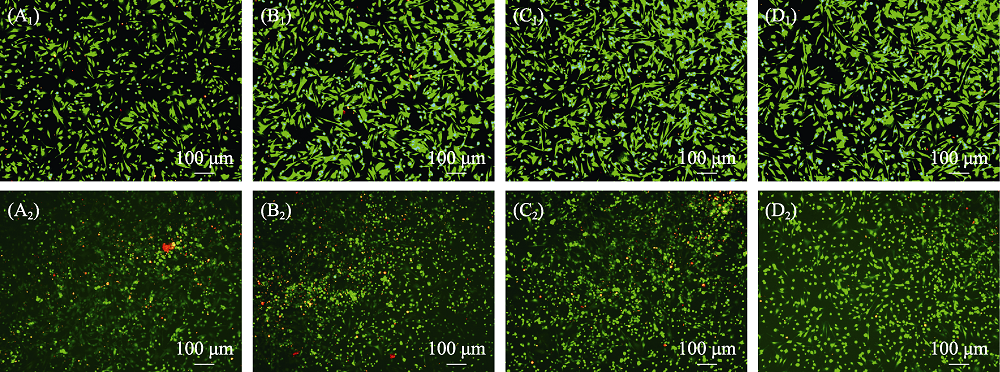
图13 (A)空白组和(B) β-TCP, (C) MS-TCP, (D) 3% Co-MS-TCP粉体处理(A1-D1)无H2O2刺激与(A2-D2) H2O2刺激的骨髓间充质干细胞的活/死Calcein-AM-PI染色荧光显微镜照片
Fig. 13 Calcein AM and PI fluorescence images of bone marrow mesenchymal stem cells treated with powders (A) Blank; (B) β-TCP; (C) MS-TCP; (D) 3% Co-MS-TCP; (A1-D1) Cells without H2O2 stimulation; (A2-D2) Cells stimulated with H2O2 showing live (green) and death (red) cells The color figures can be obtained from online edition
| [1] | LI X, LI B, SHI Y, et al. Targeting reactive oxygen species in stem cells for bone therapy. Drug Discovery Today, 2021, 5(26): 1226-1244. |
| [2] |
CERQUENI G, SCALZONE A, LICINI C, et al. Insights into oxidative stress in bone tissue and novel challenges for biomaterials. Materials Science and Engineering: C, 2021, 130(11): 112433-12.
DOI URL |
| [3] | ADJEI I M, PLUMTON G, SHARMA B. Oxidative stress and biomaterials: the inflammatory link. Oxidative Stress and Biomaterials, 2016: 89-115. |
| [4] |
LI J, DENG C, LIANG W, et al. Mn-containing bioceramics inhibit osteo-clastogenesis and promote osteoporotic bone regeneration via scavenging ROS. Bioactive Materials, 2021, 6(11): 3839-3850.
DOI URL |
| [5] | RAJULA M, PREM B, VENKATASUBBU G, et al. Nano- hydroxyapatite: a driving force for bone tissue engineering. Journal of Pharmacy & Bioallied Sciences, 2021, 13(1): S11-S14. |
| [6] |
SHI H, ZHOU Z Q, LI W D, et al. Hydroxyapatite based materials for bone tissue engineering: a brief and com-prehensive introduction. Crystals, 2021, 11(2): 149-167
DOI URL |
| [7] | XIN Z, SHUN H. Nano-hydroxyapatite and its compound in repairing bone defects. Chinese Journal of Tissue Engineering Research, 2012, 16(34): 6403-6406. |
| [8] |
RATNAYAKE J T B, MUCALO M, DIAS G J. Substituted hydroxyapatites for bone regeneration: a review of current trends. Journal Biomedical Materials Research Part B, 2017, 105 B: 1285-1299.
DOI URL |
| [9] |
OVERGAARD S, LIND M, JOSEPHSEN K, et al. Resorption of hydroxyapatite and fluorapatite ceramic coatings on weight-bearing implants: a quantitative and morphological study in dogs. Journal of Biomedical Materials Research, 2015, 39(1): 141-152.
DOI URL |
| [10] |
DEMNATI I, GROSSIN D, COMBES C, et al. A comparative physicochemical study of chlorapatite and hydroxyapatite: from powders to plasma sprayed thin coatings. Biomedical Materials, 2012, 7(5): 054101-11.
DOI URL |
| [11] |
WARIS A, DIN M, ALI A, et al. Green fabrication of Co and Co3O4 nanoparticles and their biomedical applications: a review. Open Life Sciences, 2021, 16(1): 14-30.
DOI URL |
| [12] | CAO F, ZHANG L, YOU Y, et al. An enzyme-mimicking single-atom catalyst as an efficient multiple reactive oxygen and nitrogen species scavenger for sepsis management. Angewandte Chemie International Edition, 2020, 59(13): 5146 -5153. |
| [13] |
LIU G, WANG X, ZHOU X, et al. Modulating the cobalt dose range to manipulate multisystem cooperation in bone environment: a strategy to resolve the controversies about cobalt use for orthopedic applications. Theranostics, 2020, 10(3): 1074-1089.
DOI PMID |
| [14] |
LI Y, PAN Q, XU J, et al. Overview of methods for enhancing bone regeneration in distraction osteogenesis: potential roles of biometals. Journal of Orthopaedic Translation, 2021, 27(2): 110-118.
DOI PMID |
| [15] |
USANEE P, MARCELA A O, BOCCACCINI A R. Bioactive glasses incorporating less-common ions to improve biological and physical properties. Journal of Materials Science-Materials in Medicine, 2022, 33(1): 3-41.
DOI URL |
| [16] |
SADAT S M, KHORASANI M T, DINPANAH K E, et al. Synthesis methods for nanosized hydroxyapatite with diverse structures. Acta Biomaterialia, 2013, 9(8): 7591-7621.
DOI URL |
| [17] |
TAWALARE P K, BHATKAR V B, OMANWAR S K, et al. Near- infrared emitting Ca5(PO4)3Cl:Eu2+, Nd3+ phosphor for modification of the solar spectrum. Luminescence, 2018, 33(7): 1288-1293.
DOI URL |
| [18] | KULANTHAIVEL S, ROY B, AGARWAL T, et al. Cobalt doped proangiogenic hydroxyapatite for bone tissue engineering application. Materials Science & Engineering, 2016, 58(1): 648-658. |
| [19] |
JAIRTON D, ROBERTO F, SOUZA D, et al. Ionic liquid (molten salt) phase organometallic catalysis. Chemical Reviews, 2002, 102(10): 3667-3692.
PMID |
| [20] |
LIU D, FU Q G, CHU Y H. Molten salt synthesis, formation mechanism, and oxidation behavior of nanocrystalline HfB2 powders. Journal of Advanced Ceramics, 2020, 9 (1): 35-44.
DOI URL |
| [21] |
SUCHANEK W, YASHIMA M, KAKIHANA M, et al. Hydroxyapatite ceramics with selected sintering additives. Biomaterials, 1997, 18(13): 923-933
PMID |
| [22] |
WANG Y P, YING D W, ZHU N W, et al. Improved removal of phosphorus from incinerated sewage sludge ash by thermo- chemical reduction method with CaCl2 application. Journal of Cleaner Production, 2020, 258(10): 120779-8.
DOI URL |
| [23] |
OISHI S, SUGIURA I. Growth of chlorapatite crystals from a sodium chloride flux. Bulletin of the Chemical Society of Japan, 1997, 70(10): 2483-2487.
DOI URL |
| [24] | XIE J F, LI J, KANG L Y, et al. Molten-salt-protected pyrolytic approach for fabricating borate-modified cobalt-iron spinel oxide with robust oxygen-evolving performance. ACS Sustainable Chemistry & Engineering, 2021, 9(43): 14596-14704. |
| [25] |
XIAO M, ZHANG L, LUO B, et al. Moten-salt-mediated synthesis of an atomic nickel Co-catalyst on TiO2 for improved photocatalytic H2 evolution. Angewandte Chemie International Edition, 2020, 59(18): 7230-7234.
DOI URL |
| [26] | CHRIS T. Thermodynamics of mixing of liquids in the system Ca3(PO4)2-CaCl2-CaF2-Ca(OH)2. Geochimica et Cosmochimica Acta, 1994, 58(12): 2755-2755. |
| [27] |
ZENG K, YANG X, Xie Y, et al. Molten salt pyrolysis of biomass: the evaluation of molten salt. Fuel, 2021, 302(5): 121103-10.
DOI URL |
| [28] |
PRENER J S. The growth and crystallo-graphic properties of calcium fluor- and chlorapatite crystals. Journal of the Electrochemical Society, 1967, 114(1): 77-84
DOI URL |
| [29] |
KATHLEEN M, JOCHEN L. Synthesis-structure-activity relationships in Co3O4 catalyzed CO oxidation. Frontiers in Chemistry, 2018, 6: 185-197.
DOI URL |
| [30] |
LAN Y P, SOHN H Y, MOHASSAB Y, et al. Nanoceria synthesis in the KCl-LiCl salt system: crystal formation and properties. Journal of the American Ceramic Society, 2017, 100: 1863-1875.
DOI URL |
| [31] |
MO Z Y, YANG W Y, GAO S, et al. Efficient oxygen reduction reaction by a highly porous, nitrogen-doped carbon sphere electrocatalyst through space confinement effect in nanopores. Journal of Advanced Ceramics, 2021, 10 (4): 714-728.
DOI URL |
| [32] |
ANDERSON J B, KOSTINER E. The crystal structure of cobalt-substituted calcium chlorapatite. Journal of Solid State Chemistry, 1987, 66(2): 343-349.
DOI URL |
| [33] |
LIU P, ZHONG D, XU Y, et al. Co/Fe co-doped porous graphite carbon derived from metal organic framework for microelectrolysis- fenton catalytic degradation of Rhodamine B. Journal of Environmental Chemical Engineering, 2021, 9(5): 105924-11.
DOI URL |
| [34] |
GAO Y, KONG D R, ZHANG Z Y, et al. Spinel CoMn2O4 hollow nanospheres for very wide linear and sensitive detection of hydrogen peroxide. Journal of Alloys and Compounds, 2022, 897(15): 163158-9.
DOI URL |
| [35] |
PALUSZKIEWICZ C, LÓSARCZYK A, PIJOCHA D, et al. Synthesis, structural properties and thermal stability of Mn-doped hydroxyapatite. Journal of Molecular Structure, 2010, 976(1/3): 301-309.
DOI URL |
| [36] |
AAIF A, KHALID, CHAUDHRY, et al. Zinc containing calcium phosphates obtained via microwave irradiation of suspensions. Materials Chemistry and Physics, 2022, 276(15): 124921-11.
DOI URL |
| [37] |
LIU W Y, ZUO R T, ZHU T L, et al. Forsterite-hydroxyapatite composite scaffolds with photothermal antibacterial activity for bone repair. Journal of Advanced Ceramics, 2021, 10(5): 1095-1106.
DOI URL |
| [1] | 胡佳军, 王凯, 侯鑫广, 杨婷, 夏鸿雁. 熔盐法合成高导热磷化硼及其热管理性能研究[J]. 无机材料学报, 2022, 37(9): 933-940. |
| [2] | 吴爱军, 朱敏, 朱钰方. 含铜硅酸钙纳米棒复合水凝胶用于肿瘤治疗和皮肤伤口愈合性能研究[J]. 无机材料学报, 2022, 37(11): 1203-1216. |
| [3] | 王皓轩, 刘巧沐, 王一光. 高熵过渡金属碳化物陶瓷的研究进展[J]. 无机材料学报, 2021, 36(4): 355-364. |
| [4] | 夏朝阳, 王慧, 方婧红, 张阳, 汪超越, 贺欢, 倪津崎, 石云, 李勤, 余建定. Co掺杂GaFeO3陶瓷的结构, 导电及磁性能研究[J]. 无机材料学报, 2021, 36(3): 319-324. |
| [5] | 杨言言, 李永国, 祝小雯, 杜晓, 马旭莉, 郝晓刚. 电活性镍钴双金属氧化物高选择性去除/回收水中磷酸盐离子[J]. 无机材料学报, 2021, 36(3): 292-298. |
| [6] | 金高尧, 何海传, 吴杰, 张梦源, 李亚娟, 刘又年. 锂硫电池正极用钴掺杂空心多孔碳载体材料[J]. 无机材料学报, 2021, 36(2): 203-209. |
| [7] | 张亚晨, 孟佳, 蔡坤, 盛晓晨, 乐军, 宋力昕. 基于声发射技术的Si-Cr-Ti高温抗氧化涂层弯曲失效机理研究[J]. 无机材料学报, 2021, 36(11): 1185-1192. |
| [8] | 兰青, 孙盛睿, 吴萍, 杨庆峰, 刘阳桥. 钴掺杂氧化铜/可见光协同活化PMS降解罗丹明B及其机理研究[J]. 无机材料学报, 2021, 36(11): 1171-1177. |
| [9] | 刘强, 丁杰, 纪国敬, 胡绢敏, 顾浩, 钟秦. Fe-Co-K/ZrO2催化CO2加氢制低碳烯烃[J]. 无机材料学报, 2021, 36(10): 1053-1058. |
| [10] | 王举汉,文雄,刘成超,张煜华,赵燕熹,李金林. 多级孔Co/Al-SiO2催化剂制备及其费-托合成催化性能[J]. 无机材料学报, 2020, 35(9): 999-1004. |
| [11] | 柏祥涛,班丽卿,庄卫东. 高镍三元正极材料的包覆与掺杂改性研究进展[J]. 无机材料学报, 2020, 35(9): 972-986. |
| [12] | 吕子夜, 唐谊平, 曹华珍, 郑国渠, 侯广亚. V掺杂对Ni-Co-S/细菌纤维素基碳气凝胶电催化性能的影响[J]. 无机材料学报, 2020, 35(10): 1142-1148. |
| [13] | 张晓锋,张冠华,孟跃,薛继龙,夏盛杰,倪哲明. 席夫碱钴改性CoCr-LDHs材料光催化降解亚甲基蓝研究[J]. 无机材料学报, 2019, 34(9): 974-982. |
| [14] | 王巧, 周庆, 安爽, 杨浩, 黄利宏. 铁助剂对乙酸自热重整制氢用CoxAl3FeyOm±δ催化剂的影响[J]. 无机材料学报, 2019, 34(8): 811-816. |
| [15] | 柯剑煌, 谢凯, 韩喻, 孙巍巍, 罗世强, 刘锦锋. 基于不同共溶剂体系对于高电压正极材料LiCoPO4的形貌控制[J]. 无机材料学报, 2019, 34(6): 618-624. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||