无机材料学报 ›› 2021, Vol. 36 ›› Issue (12): 1316-1322.DOI: 10.15541/jim20210125
所属专题: 【生物材料】骨骼与齿类组织修复
收稿日期:2021-03-03
修回日期:2021-04-07
出版日期:2021-12-20
网络出版日期:2021-04-30
通讯作者:
王佐林, 教授. E-mail: zuolin@tongji.edu.cn
作者简介:张大卫(1994-), 男, 硕士研究生. E-mail: david_zhang@tongji.edu.cn
基金资助:
ZHANG Dawei1( ), ZHU Liyuan2, LU Hongliang2, WANG Zuolin1(
), ZHU Liyuan2, LU Hongliang2, WANG Zuolin1( )
)
Received:2021-03-03
Revised:2021-04-07
Published:2021-12-20
Online:2021-04-30
Contact:
WANG Zuolin, professor. E-mail: zuolin@tongji.edu.cn
About author:ZHANG Dawei(1994-), male, Master candidate. E-mail: david_zhang@tongji.edu.cn
Supported by:摘要:
种植体周炎会导致种植体周牙龈附着丧失、骨组织吸收, 甚至种植体松动、脱落。本研究采用原子层沉积技术联合硅烷化修饰在钛表面制备氧化锌纳米薄膜和纤连蛋白复合涂层。选取种植体周炎相关致病菌伴放线聚集杆菌和牙龈卟啉单胞菌用作该复合涂层抗菌效果的体外验证。同时, 检测牙龈成纤维细胞在钛、氧化锌修饰钛及纤连蛋白与氧化锌复合修饰钛表面黏附、增殖及其相关基因的表达。在大鼠上颌第一磨牙位点通过注射细菌法建立种植体周炎模型, 并比较不同材料的钛钉周围骨破坏范围和牙龈组织中的炎症程度。实验结果显示, 该复合涂层对伴放线聚集杆菌和牙龈卟啉单胞菌的24 h抗菌率分别为80.9%和75.7%; 牙龈成纤维细胞在复合修饰钛片表面展现出良好的黏附、增殖状态; 在大鼠种植体周炎模型中, Micro-CT结果显示, 纯钛钉周骨组织吸收为(1.14±0.71) mm, 而经复合修饰的钛钉周围骨组织吸收仅为(0.37±0.28) mm。石蜡切片观察和qPCR定量检测结果显示, 纯钛钉周牙龈及牙槽骨组织的炎症反应较重, 而经复合修饰的钛钉周围组织炎症反应明显减轻。因此, 该复合修饰可能在牙种植体防御细菌入侵、增强牙龈附着和降低牙龈炎症反应方面发挥作用。
中图分类号:
张大卫, 朱立远, 卢红亮, 王佐林. 钛表面氧化锌与纤连蛋白复合修饰应用于抗种植体周炎的研究[J]. 无机材料学报, 2021, 36(12): 1316-1322.
ZHANG Dawei, ZHU Liyuan, LU Hongliang, WANG Zuolin. Titanium Modified with ZnO Nanofilm and Fibronectin: Preventing Peri-implantitis and Biocompatibility[J]. Journal of Inorganic Materials, 2021, 36(12): 1316-1322.

图1 (a) Ti/ZnO和Ti/ZnO/Fn的制备过程示意图, (b, e) Ti、(c,f) Ti/ZnO和(d,g) Ti/ZnO/Fn的SEM(b~d)低倍照片及 (e~g) 相应的高倍照片
Fig. 1 (a) Schematic diagram of fabrication progress of Ti/ZnO and Ti/ZnO/Fn, and SEM images of (b, e) Ti, (c, f) Ti/ZnO and (d, g) Ti/ZnO/Fn at low (b-d) and high (e-g) magnification ALD: Atomic layer deposition
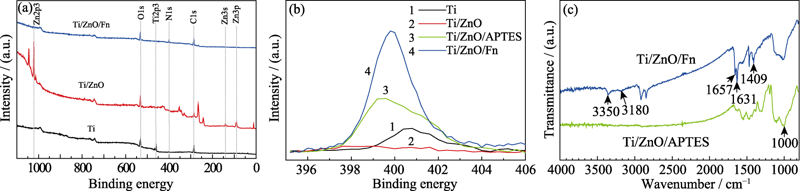
图2 (a)Ti、Ti/ZnO和Ti/ZnO/Fn的XPS全谱, (b)Ti、Ti/ZnO、Ti/ZnO/APTES和Ti/ZnO/Fn的N1s窄谱, 以及(c)Ti/ZnO/APTES和Ti/ZnO/Fn的FT-IR光谱图
Fig. 2 (a) XPS spectra of Ti, Ti/ZnO, and Ti/ZnO/Fn, (b) high-resolution spectra of N1s peak of Ti, Ti/ZnO, Ti/ZnO/APTES, and Ti/ZnO/Fn, and (c) FT-IR spectra of Ti/ZnO/APTES and Ti/ZnO/Fn Ti/ZnO/APTES: Ti disc with ZnO nanofilm modified with APTES
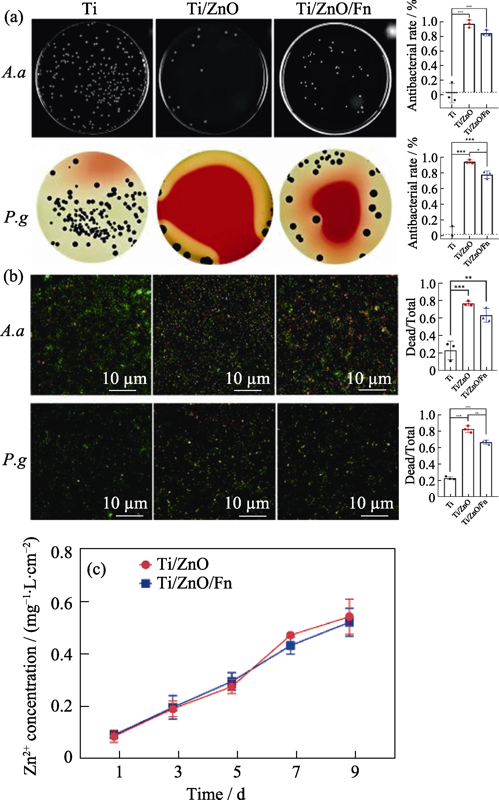
图3 复合材料的24 h抗A. a和P. g 效果及其机理
Fig. 3 Antibacterial effects and mechanisms of Ti, Ti/ZnO, and Ti/ZnO/Fn against A. a and P. g for 24 h (a) Typical bacteria colonies of A. a and P. g on Ti, Ti/ZnO, and Ti/ZnO/Fn (left) with average corresponding antibacterial rates (right); (b) Fluorecent images of dead bacteria (red) and total bacteria (green) on surfaces of Ti, Ti/ZnO, and Ti/ZnO/Fn after incubation (left) and their dead/total ratio (right); (c) Cumulative zinc ion release curves of Ti/ZnO and Ti/ZnO/Fn A. a: Aggregatibacter actinomycetemcomitans; P. g: Porphyro-monas gingivalis. The error bars indicate standard deviations. *P < 0.05, **P < 0.01, and ***P < 0.005 (n=3, One-way ANOVA)
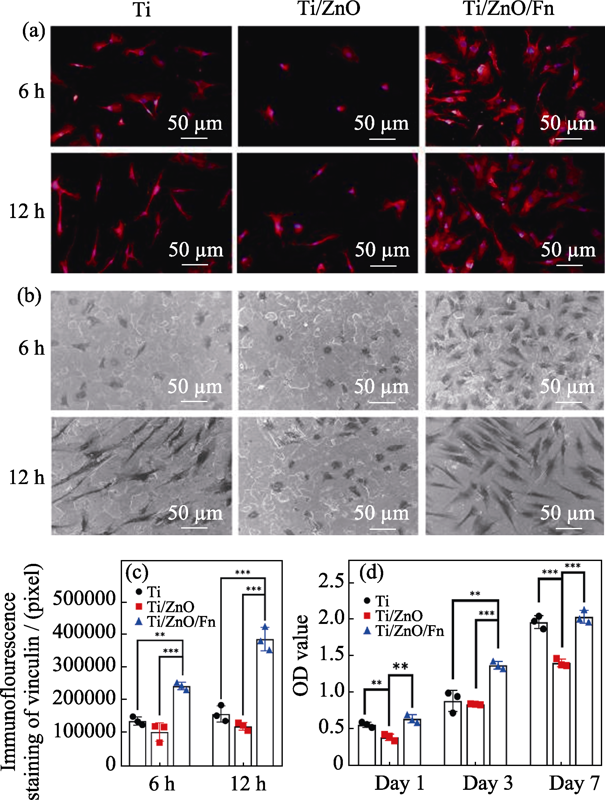
图4 复合材料的细胞相容性
Fig. 4 Cytocompatibility of Ti/ZnO/Fn on human gingival fibroblasts (a) Fluorescent images of hGFs cultured on Ti, Ti/ZnO and Ti/ZnO/Fn for 6 and 12 h with vinculin staining (red) and nuclei staining (blue); (b) SEM images of hGFs attachment on Ti, Ti/ZnO and Ti/ZnO/Fn for 6 and 12 h; (c) Quantitative measurement of spreading area of hGFs on Ti, Ti/ZnO and Ti/ZnO/Fn after 6 and 12 h culture; (d) Cell viabilities cultured on Ti, Ti/ZnO and Ti/ZnO/Fn after 1, 3 and 7 d hGFs: human gingival fibroblasts. Error bars indicate standard deviations. **P < 0.01, and ***P < 0.005 (n=3, One-way ANOVA)
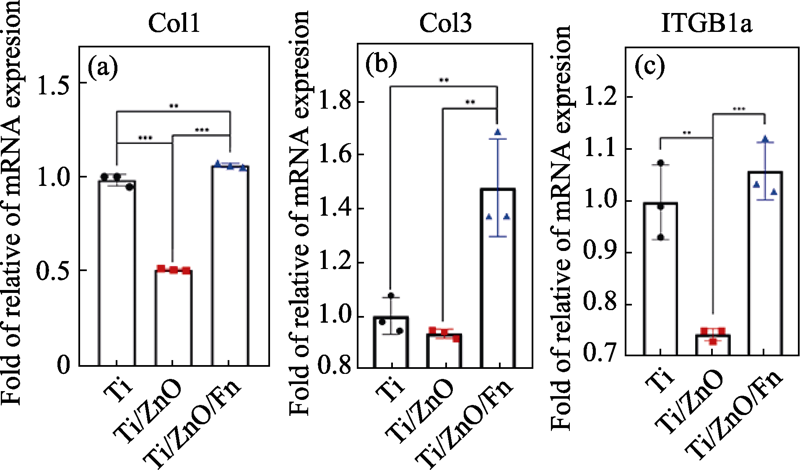
图5 hGFs在Ti、Ti/ZnO和Ti/ZnO/Fn表面培养3 d后(a) Col1、(b)Col3和(c)ITGB1a基因的相对表达量
Fig. 5 Quantification of the gene expression of (a) Col1, (b) Col3 and (c) ITGB1a in hGFs on Ti, Ti/ZnO and Ti/ZnO/Fn for 3 d by qPCR Data are normalized against reference gene expression of glyceraldehyde- 3-phosphate dehydrogenase and standardized with Ct (cycle threshold) of Ti. Error bars indicate standard deviations. **P < 0.01 and ***P < 0.005 (n=3, One-way ANOVA)
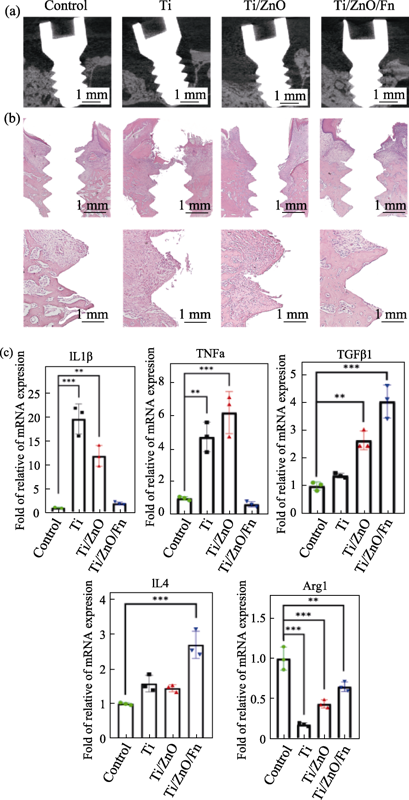
图6 Ti、Ti/ZnO和Ti/ZnO/Fn制成的钛钉植入大鼠牙槽骨4 w后(a)Micro-CT矢状面截图, (b)钛钉周组织石蜡切片H&E染色照片, 和(c)钛钉周牙龈组织中IL1β、Arg1、TNFα、IL4、TGFβ1基因的相对表达量
Fig. 6 (a) Sagittal pictures of Micro-CT, (b) H&E-stained tissues around screws, and (c) quantification of the gene expression of IL1β, Arg1, TNFα, IL4, and TGFβ1 by qPCR in gingiva tissues around Ti, Ti/ZnO and Ti/ZnO/Fn screws after being inserted into maxillae of rats for 4 w Data are normalized against reference gene expression of glyceraldehyde-3-phosphate dehydrogenase and standardized with Ct of control. Control: Ti screws infused with bacteria culture medium; Ti, Ti/ZnO and Ti/ZnO/Fn: Ti, Ti/ZnO and Ti/ZnO/Fn screws infused with mixed bacterial suspension. Error bars indicate standard deviations. *P < 0.05, **P < 0.01, and ***P < 0.005 (n=3, One-way ANOVA)
| Gene | Forward primer sequence (5′-3′) | Reverse primer sequence (3′-5′) |
|---|---|---|
| GAPDH | GCACCGTCAAGGCTGAGAAC | TGGTGAAGACGCCAGTGGA |
| Col1 | TCTAGACATGTTCAGCTTTGTGGAC | TCTGTACGCAGGTGATTGGTG |
| Col3 | GCAAATTCACCTACACAGTTCTGGA | CTTGATCAGGACCACCAATGTCATA |
| ITGB1α | TGTGTCAGACCTGCCTTGGTG | AGGAACATTCCTGTGTGCATGTG |
表S1 人牙龈成纤维细胞相关基因正反引物序列
Table S1 Specific forward and reverse primer sequences of detected genes of HGFs
| Gene | Forward primer sequence (5′-3′) | Reverse primer sequence (3′-5′) |
|---|---|---|
| GAPDH | GCACCGTCAAGGCTGAGAAC | TGGTGAAGACGCCAGTGGA |
| Col1 | TCTAGACATGTTCAGCTTTGTGGAC | TCTGTACGCAGGTGATTGGTG |
| Col3 | GCAAATTCACCTACACAGTTCTGGA | CTTGATCAGGACCACCAATGTCATA |
| ITGB1α | TGTGTCAGACCTGCCTTGGTG | AGGAACATTCCTGTGTGCATGTG |
| Gene | Forward primer sequence (5′-3′) | Reverse primer sequence (3′-5′) |
|---|---|---|
| GAPDH | CCCCAATGTATCCGTTGTG | CTCAGTGTAGCCCAGGATGC |
| IL1β | GACCTGTTCTTTGAGGCTGACA | CTCATCTGGACAGCCCAAGTC |
| Arg1 | AGCAGAGACCCAGAAGAATG | TTTCCTTTCAGTTCCTTCAG |
| TNFα | GACAAGGCTGCCCCGACTAT | GGGAGACTCCTCCCAGGTACA |
| IL4 | TCGCTTGCCTTGGTGGTC | TGTGATGTTGCTCAGCTCCTC |
| TGFβ1 | AGGACCTGGGTTGGAAGTGG | AGTTGGCATGGTAGCCCTTG |
表S2 大鼠炎症相关基因正反引物序列
Table S2 Specific forward and reverse primer sequences of detected genes of rat
| Gene | Forward primer sequence (5′-3′) | Reverse primer sequence (3′-5′) |
|---|---|---|
| GAPDH | CCCCAATGTATCCGTTGTG | CTCAGTGTAGCCCAGGATGC |
| IL1β | GACCTGTTCTTTGAGGCTGACA | CTCATCTGGACAGCCCAAGTC |
| Arg1 | AGCAGAGACCCAGAAGAATG | TTTCCTTTCAGTTCCTTCAG |
| TNFα | GACAAGGCTGCCCCGACTAT | GGGAGACTCCTCCCAGGTACA |
| IL4 | TCGCTTGCCTTGGTGGTC | TGTGATGTTGCTCAGCTCCTC |
| TGFβ1 | AGGACCTGGGTTGGAAGTGG | AGTTGGCATGGTAGCCCTTG |
| Sample | C1s/% | O1s/% | N1s/% | Si2p3/% | Ti2p3/% | Zn2p3/% |
|---|---|---|---|---|---|---|
| Ti | 57.69 | 31.07 | 1.92 | 0.02 | 9.26 | 0.05 |
| Ti/ZnO | 38.94 | 37.22 | 2.26 | 0.08 | 1.07 | 20.44 |
| Ti/ZnO/Fn | 63.91 | 21.2 | 11.88 | 2.27 | 0.13 | 0.6 |
表S3 Ti、Ti/ZnO和Ti/ZnO/Fn的表面元素含量
Table S3 Elemental chemical composition of Ti, Ti/ZnO and Ti/ZnO/Fn
| Sample | C1s/% | O1s/% | N1s/% | Si2p3/% | Ti2p3/% | Zn2p3/% |
|---|---|---|---|---|---|---|
| Ti | 57.69 | 31.07 | 1.92 | 0.02 | 9.26 | 0.05 |
| Ti/ZnO | 38.94 | 37.22 | 2.26 | 0.08 | 1.07 | 20.44 |
| Ti/ZnO/Fn | 63.91 | 21.2 | 11.88 | 2.27 | 0.13 | 0.6 |
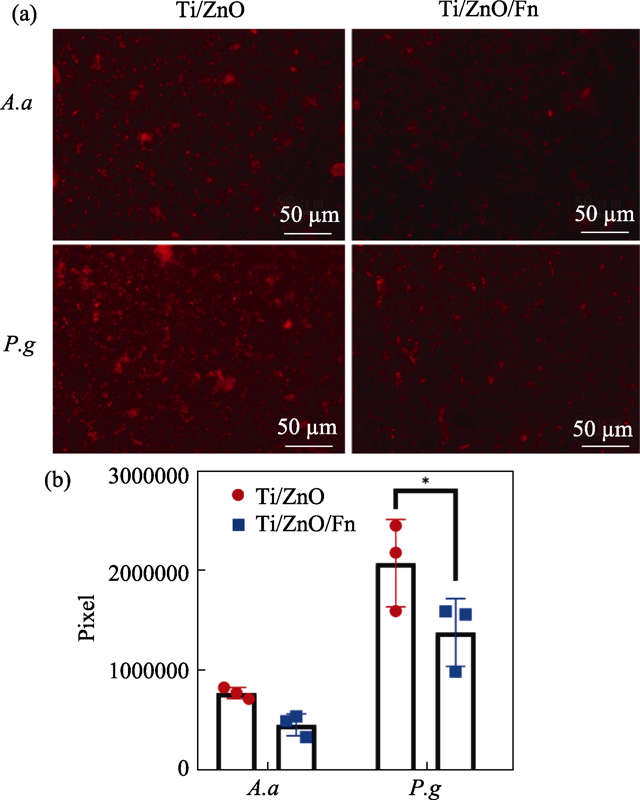
图S1 使用荧光探针标记的在材料表面培养24 h细菌胞内ROS及其含量
Fig. S1 Reactive oxygen species (ROS) detected by fluorescence probes in bacteria cultured on materials for 24 h (a) Fluorescent images of ROS in A. a and P. g cultured on Ti/ZnO and Ti/ZnO/Fn; (b) Quantitative measurement of area of ROS in A. a and P. g Error bars indicate standard deviations: *P < 0.05 (n=3, Student’s t test)
| [1] |
SIMONIS PIERRE, DUFOUR THOMAS, TENENBAUM HENRI. Long-term implant survival and success: a 10-16-year follow-up of non-submerged dental implants. Clin. Oral Implants Res., 2010, 21(7): 772-777.
DOI URL |
| [2] |
DIXON DOUGLAS R, LONDON ROBERT M. Restorative design and associated risks for peri-implant diseases. Periodontol 2000, 2019, 81(1): 167-178.
DOI URL |
| [3] |
DAUBERT DIANE M, WEINSTEIN BRADLEY F. Biofilm as a risk factor in implant treatment. Periodontol 2000, 2019, 81(1): 29-40.
DOI URL |
| [4] |
DE WAAL Y C, EIJSBOUTS H V, WINKEL E G, et al. Microbial characteristics of peri-implantitis: a case-control study. J. Periodontol, 2017, 88(2): 209-217.
DOI URL |
| [5] |
DE AVILA ERICA DORIGATTI, LIMA BRUNO P, SEKIYA TAKEO, et al. Effect of UV-photofunctionalization on oral bacterial attachment and biofilm formation to titanium implant material. Biomaterials, 2015, 67: 84-92.
DOI URL |
| [6] |
SOBOLEV ALEXANDER, VALKOV ANTON, KOSSENKO ALEXEY, et al. Bioactive coating on Ti alloy with high osseointegration and antibacterial nanoparticles. ACS Appl. Mater. Interfaces, 2019, 11(43): 39534-39544.
DOI URL |
| [7] |
YUAN ZHANG, TAO BAILONG, HE YE, et al. Remote eradication of biofilm on titanium implant via near-infrared light triggered photothermal/photodynamic therapy strategy. Biomaterials, 2019, 223: 119479.
DOI URL |
| [8] |
ALQATTAN M, PETERS L, YANG F, et al. Microstructure, mechanical behaviour and antibacterial activity of biomedical Ti-xMn-yCu alloys. J. Alloys Compd., 2021, 856: 158165.
DOI URL |
| [9] |
DIEFENBECK M, SCHRADER C, GRAS F, et al. Gentamicin coating of plasma chemical oxidized titanium alloy prevents implant-related osteomyelitis in rats. Biomaterials, 2016, 101: 156-164.
DOI URL |
| [10] |
LI BAOE, ZHANG LEI, WANG DONGHUI, et al. Thermosensitive-hydrogel-coated titania nanotubes with controlled drug release and immunoregulatory characteristics for orthopedic applications. Mat. Sci. Eng. C-Mater., 2021, 122: 111878.
DOI URL |
| [11] |
KUMAR RAJESH, UMAR AHMAD, KUMAR GIRISHE, et al. Antimicrobial properties of ZnO nanomaterials: a review. Ceram. Int., 2017, 43(5): 3940-3961.
DOI URL |
| [12] | YU FEN, FANG XUAN, JIA HUIMIN, et al. Zn or O? An atomic level comparison on antibacterial activities of zinc oxides. Chemistry, 2016, 22(24): 8053-8058. |
| [13] | ZHU LIYUAN, YUAN KAIPING, YANG JIAHE, et al. Hierarchical highly ordered SnO2 nanobowl branched ZnO nanowires for ultrasensitive and selective hydrogen sulfide gas sensing. Microsystems & Nanoengineering, 2020, 6(1): 30-43. |
| [14] |
JIAN SHENGRUI, LEE YAHUI. Nanoindentation-induced interfacial fracture of ZnO thin films deposited on Si(111) substrates by atomic layer deposition. J. Alloys Compd., 2014, 587: 313-317.
DOI URL |
| [15] |
TAPILY K, GU D, BAUMGART H, et al. Mechanical and structural characterization of atomic layer deposition-based ZnO films. Semicond. Sci. Tech., 2011, 26(11): 115005.
DOI URL |
| [16] |
LUO QIMING, CAO HUILIANG, WANG LANYU, et al. ZnO@ZnS nanorod-array coated titanium: good to fibroblasts but bad to bacteria. J. Colloid. Interface Sci., 2020, 579: 50-60.
DOI URL |
| [17] | BAKHORI SITI KHADIJAH MOHD, MAHMUD SHAHROM, MASUDI SAM’AN MALIK, et al. Cytotoxicity evaluation of ZnO-eugenol (ZOE) using different ZnO structure on human gingival fibroblast. AIP Conference Proceedings, 2017, 1865(1): 020008. |
| [18] |
JORDAHL STACY, SOLORIO LUIS, NEALE DYLAN B, et al. Engineered fibrillar fibronectin networks as three-dimensional tissue scaffolds. Adv. Mater., 2019, 31(46): 1904580.
DOI URL |
| [19] |
MCWHORTER FRANCES Y, WANG TINGTING, NGUYEN PHOEBE, et al. Modulation of macrophage phenotype by cell shape. Proc. Natl. Acad. Sci. U S A, 2013, 110(43): 17253-17258.
DOI URL |
| [20] |
LI JUN, TAN LEI, LIU XIANGMEI, et al. Balancing bacteria-osteoblast competition through selective physical puncture and biofunctionalization of ZnO/polydopamine/arginine- glycine-aspartic acid-cysteine nanorods. ACS Nano, 2017, 11(11): 11250-11263.
DOI URL |
| [21] |
JIAN XIAOCHONG, HUANG WENXIU, WU DONG, et al. Effect of fibronectin-coated micro-grooved titanium surface on alignment, adhesion, and proliferation of human gingival fibroblasts. Med. Sci. Monitor., 2017, 23: 4749-4759.
DOI URL |
| [22] |
KRÓL A, POMASTOWSKI P, RAFIŃSKA K, et al. Zinc oxide nanoparticles: synthesis, antiseptic activity and toxicity mechanism. Adv. Colloid. Interface Sci., 2017, 249: 37-52.
DOI URL |
| [23] |
KOUTOUZIS THEOFILOS, EASTMAN CHRISTIE, CHUKKAPALLI SASANKA, et al. A novel rat model of polymicrobial peri-implantitis: a preliminary study. J. Periodontol., 2017, 88(2): e32-e41.
DOI URL |
| [24] |
YAMAZAKI SEIYA, MASAKI CHIHIRO, NODAI TOMOTAKA, et al. The effects of hyperglycaemia on peri-implant tissues after osseointegration. J. Prosthodont Res., 2020, 64(2): 217-223.
DOI URL |
| [1] | 张万文, 罗建强, 刘淑娟, 马建国, 张小平, 杨松旺. 氧化锆间隔层的低温喷涂制备及其三层结构钙钛矿太阳能电池应用性能[J]. 无机材料学报, 2023, 38(2): 213-218. |
| [2] | 柳琪, 朱璨, 谢贵震, 王俊, 张东明, 邵刚勤. Ce掺杂SrMgF4超结构多晶体的吸收/光致发光光谱[J]. 无机材料学报, 2022, 37(8): 897-902. |
| [3] | 薛虹云, 王聪宇, MAHMOOD Asad, 于佳君, 王焱, 谢晓峰, 孙静. 二维g-C3N4与Ag-TiO2复合光催化剂降解气态乙醛抗失活研究[J]. 无机材料学报, 2022, 37(8): 865-872. |
| [4] | 文志勤, 黄彬荣, 卢涛仪, 邹正光. 压力对PbTiO3结构和热物性质影响的第一性原理研究[J]. 无机材料学报, 2022, 37(7): 787-794. |
| [5] | 迟聪聪, 屈盼盼, 任超男, 许馨, 白飞飞, 张丹洁. SiO2@Ag@SiO2@TiO2核壳结构的制备及其光催化降解性能[J]. 无机材料学报, 2022, 37(7): 750-756. |
| [6] | 黄郅航, 滕官宏伟, 铁鹏, 范德松. 钙钛矿陶瓷薄膜的电致变色特性[J]. 无机材料学报, 2022, 37(6): 611-616. |
| [7] | 焦博新, 刘兴翀, 全子威, 彭永姗, 周若男, 李海敏. L-精氨酸掺杂钙钛矿太阳电池性能研究[J]. 无机材料学报, 2022, 37(6): 669-675. |
| [8] | 杨慧平, 周学凡, 方豪杰, 张晓云, 罗行, 张斗. 钛酸铋钠基无铅铁电陶瓷场致应变性能研究[J]. 无机材料学报, 2022, 37(6): 603-610. |
| [9] | 林啊鸣, 孙宜阳. Cs2SnI6低指数晶面稳定性的第一性原理计算研究[J]. 无机材料学报, 2022, 37(6): 691-696. |
| [10] | 魏子钦, 夏翔, 李勤, 李国荣, 常江. 钛酸钡/硅酸钙复合生物活性压电陶瓷的制备及性能研究[J]. 无机材料学报, 2022, 37(6): 617-622. |
| [11] | 马慧, 陶疆辉, 王艳妮, 韩玉, 王亚斌, 丁秀萍. 硅钛杂化介孔球负载金纳米粒子及其催化性能调控[J]. 无机材料学报, 2022, 37(4): 404-412. |
| [12] | 张国庆, 秦鹏, 黄富强. 空间限域铅离子与钙钛矿纳米晶间的可逆转换与信息存储应用[J]. 无机材料学报, 2022, 37(4): 445-451. |
| [13] | 张枫娟, 韩博宁, 曾海波. 钙钛矿量子点光伏与荧光聚光电池: 现状与挑战[J]. 无机材料学报, 2022, 37(2): 117-128. |
| [14] | 明月, 胡玥, 梅安意, 荣耀光, 韩宏伟. 醋酸铅添加剂在印刷钙钛矿太阳能电池中的应用[J]. 无机材料学报, 2022, 37(2): 197-203. |
| [15] | 王万海, 周杰, 唐卫华. 钙钛矿薄膜缺陷调控策略在太阳能电池中的应用[J]. 无机材料学报, 2022, 37(2): 129-139. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||