无机材料学报 ›› 2022, Vol. 37 ›› Issue (1): 65-71.DOI: 10.15541/jim20210192
收稿日期:2021-03-24
修回日期:2021-04-23
出版日期:2022-01-20
网络出版日期:2021-11-12
通讯作者:
袁 霞, 教授. E-mail: yuanxia@xtu.edu.cn
作者简介:陈小梅(1996-), 女, 硕士研究生. E-mail: cxm1077@163.com
基金资助:
CHEN Xiaomei( ), CHEN Ying, YUAN Xia(
), CHEN Ying, YUAN Xia( )
)
Received:2021-03-24
Revised:2021-04-23
Published:2022-01-20
Online:2021-11-12
Contact:
YUAN Xia, professor. E-mail: yuanxia@xtu.edu.cn
About author:CHEN Xiaomei(1996-), female, Master candidate. E-mail: cxm1077@163.com
Supported by:摘要:
环己基过氧化氢(CHHP)分解是环己烷无催化氧化工艺制备环己醇和环己酮的重要反应步骤。本研究以Co3O4纳米颗粒为内核, 十六烷基三甲基溴化铵(CTAB)为模板剂, 正硅酸四乙酯(TEOS)为硅源, 采用模板法制备了核壳结构材料Co3O4@SiO2。考察了SiO2壳层制备条件: 乙醇和水的比例、CTAB的浓度和TEOS的用量对核壳材料结构的影响。使用不同技术手段表征材料的结构特征, 在CHHP分解反应中进行材料的催化性能评价和稳定性考察。结果表明, 壳层薄且孔隙率高的材料催化性能更好, 并且核壳结构可以减少钴元素的流失, 催化材料在回收使用过程中出现了不同程度的壳层破碎现象。
中图分类号:
陈小梅, 陈颖, 袁霞. 核壳材料Co3O4@SiO2催化环己基过氧化氢分解[J]. 无机材料学报, 2022, 37(1): 65-71.
CHEN Xiaomei, CHEN Ying, YUAN Xia. Decomposition of Cyclohexyl Hydroperoxide Catalyzed by Core-shell Material Co3O4@SiO2[J]. Journal of Inorganic Materials, 2022, 37(1): 65-71.
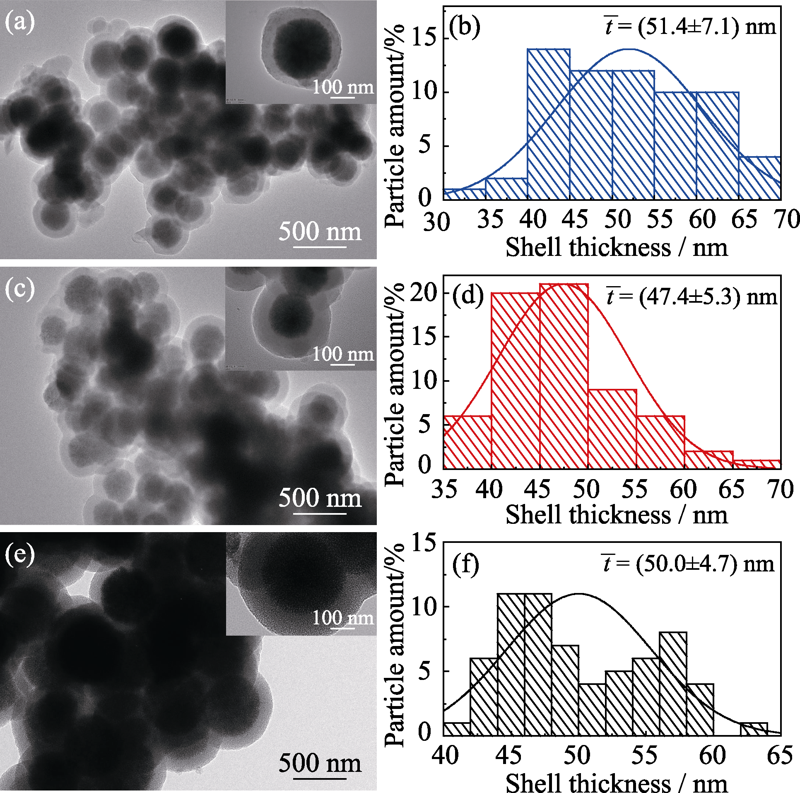
图4 不同CTAB浓度制备的Co3O4@SiO2的TEM照片及其壳层厚度统计图
Fig. 4 TEM images and shell thickness statistics of Co3O4@SiO2 prepared with different concentrations of CTAB (a, b) 5 mmol/L; (c, d) 14 mmol/L; (e, f) 25 mmol/L
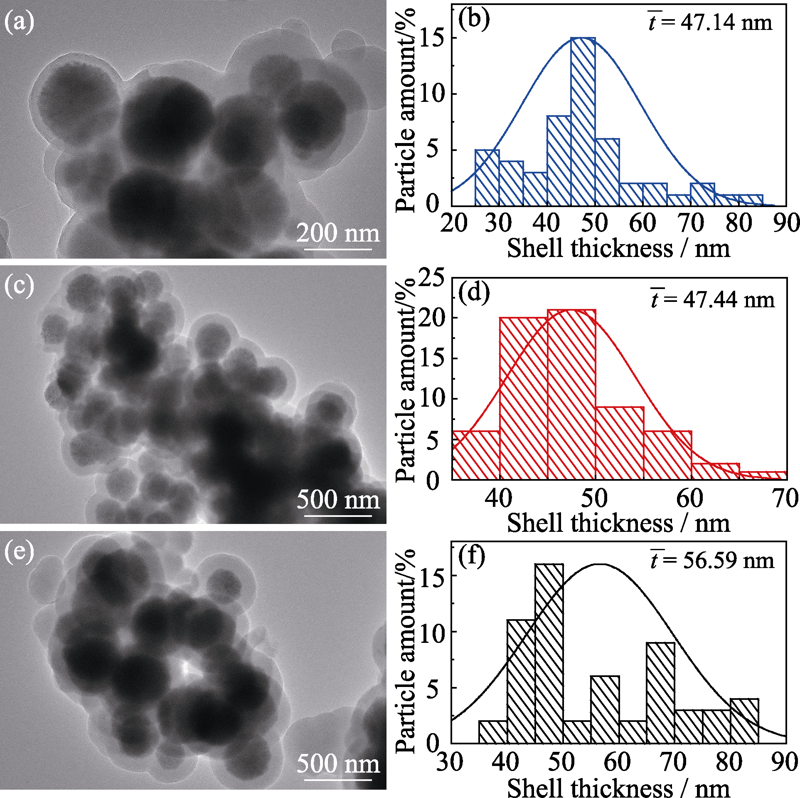
图 5 不同TEOS用量制备的Co3O4@SiO2的TEM照片及其壳层厚度统计图
Fig. 5 TEM images and shell thickness statistics of Co3O4@SiO2 prepared with different TEOS dosages (a, b) 0.8 mL; (c, d) 1.2 mL; (e, f) 2.0 mL
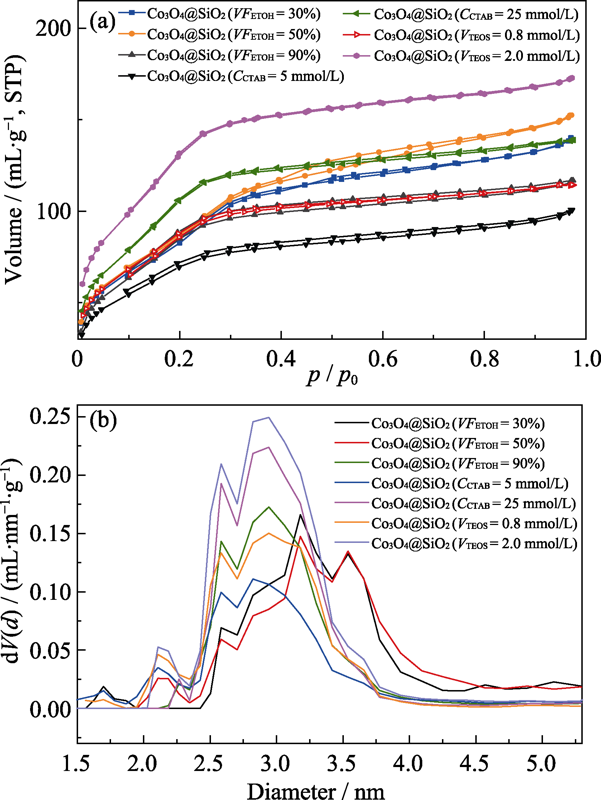
图6 催化材料的N2物理吸脱附等温线(a)和孔径分布图(b)
Fig. 6 N2 physical adsorption and desorption isotherms (a) and pore size distribution (b) of Co3O4@SiO2 Colorful figures are available on website
| Sample | nCTAB/nTEOS | SBET/(m2∙g-1) | Dpore/nm | Vpore/(cm3∙g-1) |
|---|---|---|---|---|
| Co3O4@SiO2 (VFETOH = 30%) | 2.56 | 332 | 3.18 | 0.22 |
| Co3O4@SiO2 (VFETOH = 50%) | 2.56 | 334 | 3.18 | 0.24 |
| Co3O4@SiO2 (VFETOH = 90%) | 2.56 | 319 | 2.94 | 0.18 |
| Co3O4@SiO2 (CCTAB = 5 mmol/L) | 0.93 | 254 | 2.82 | 0.16 |
| Co3O4@SiO2 (CCTAB = 25 mmol/L) | 4.67 | 396 | 2.94 | 0.21 |
| Co3O4@SiO2 (VTEOS = 0.8 mL) | 3.84 | 327 | 2.94 | 0.18 |
| Co3O4@SiO2 (VTEOS = 2.0 mL) | 1.54 | 486 | 2.94 | 0.27 |
表1 催化材料的物理结构特性
Table 1 Physical structural properties of catalytic materials
| Sample | nCTAB/nTEOS | SBET/(m2∙g-1) | Dpore/nm | Vpore/(cm3∙g-1) |
|---|---|---|---|---|
| Co3O4@SiO2 (VFETOH = 30%) | 2.56 | 332 | 3.18 | 0.22 |
| Co3O4@SiO2 (VFETOH = 50%) | 2.56 | 334 | 3.18 | 0.24 |
| Co3O4@SiO2 (VFETOH = 90%) | 2.56 | 319 | 2.94 | 0.18 |
| Co3O4@SiO2 (CCTAB = 5 mmol/L) | 0.93 | 254 | 2.82 | 0.16 |
| Co3O4@SiO2 (CCTAB = 25 mmol/L) | 4.67 | 396 | 2.94 | 0.21 |
| Co3O4@SiO2 (VTEOS = 0.8 mL) | 3.84 | 327 | 2.94 | 0.18 |
| Co3O4@SiO2 (VTEOS = 2.0 mL) | 1.54 | 486 | 2.94 | 0.27 |
| Entry | Sample | Co content/% |
|---|---|---|
| 1 | Co3O4@SiO2 (VFETOH = 90%) | 39.9 |
| 2 | Co3O4@SiO2 (CCTAB = 5 mmol/L) | 40.1 |
| 3 | Co3O4@SiO2 (CCTAB = 25 mmol/L) | 39.3 |
| 4 | Co3O4@SiO2 (VTEOS = 0.8 mL) | 47.8 |
| 5 | Co3O4@SiO2 (VTEOS = 2.0 mL) | 30.6 |
表2 催化材料的Co元素含量
Table 2 Co content of catalytic materials
| Entry | Sample | Co content/% |
|---|---|---|
| 1 | Co3O4@SiO2 (VFETOH = 90%) | 39.9 |
| 2 | Co3O4@SiO2 (CCTAB = 5 mmol/L) | 40.1 |
| 3 | Co3O4@SiO2 (CCTAB = 25 mmol/L) | 39.3 |
| 4 | Co3O4@SiO2 (VTEOS = 0.8 mL) | 47.8 |
| 5 | Co3O4@SiO2 (VTEOS = 2.0 mL) | 30.6 |
| Catalytic material | Con./% | Selectivity/% | ||||
|---|---|---|---|---|---|---|
| A | K | Acid | Ester | A+K | ||
| Blank | 11.58 | 162.27 | 36.16 | 12.56 | 14.40 | 198.43 |
| Co3O4 | 86.83 | 69.15 | 38.08 | 6.73 | -15.63 | 107.23 |
| Co3O4@SiO2 (VFETOH = 30%) | 41.54 | 66.93 | 37.70 | 8.14 | -26.54 | 104.62 |
| Co3O4@SiO2 (VFETOH = 50%) | 60.44 | 67.73 | 32.87 | 7.33 | -18.46 | 100.60 |
| Co3O4@SiO2 (VFETOH = 90%) | 52.62 | 64.58 | 30.20 | 8.54 | -25.15 | 94.79 |
| Co3O4@SiO2 (CCTAB = 5 mmol/L) | 55.62 | 74.72 | 36.68 | 6.59 | -20.69 | 111.40 |
| Co3O4@SiO2 (CCTAB =25 mmol/L) | 66.68 | 72.18 | 31.01 | 6.83 | -15.76 | 103.19 |
| Co3O4@SiO2 (VTEOS = 0.8 mL) | 81.68 | 70.60 | 34.06 | 7.12 | -15.97 | 104.65 |
| Co3O4@SiO2 (VTEOS = 2.0 mL) | 73.55 | 64.75 | 27.90 | 6.28 | -18.74 | 92.64 |
表3 催化材料在CHHP分解反应中的催化性能
Table 3 Catalytic performance of catalytic materials in CHHP decomposition reaction
| Catalytic material | Con./% | Selectivity/% | ||||
|---|---|---|---|---|---|---|
| A | K | Acid | Ester | A+K | ||
| Blank | 11.58 | 162.27 | 36.16 | 12.56 | 14.40 | 198.43 |
| Co3O4 | 86.83 | 69.15 | 38.08 | 6.73 | -15.63 | 107.23 |
| Co3O4@SiO2 (VFETOH = 30%) | 41.54 | 66.93 | 37.70 | 8.14 | -26.54 | 104.62 |
| Co3O4@SiO2 (VFETOH = 50%) | 60.44 | 67.73 | 32.87 | 7.33 | -18.46 | 100.60 |
| Co3O4@SiO2 (VFETOH = 90%) | 52.62 | 64.58 | 30.20 | 8.54 | -25.15 | 94.79 |
| Co3O4@SiO2 (CCTAB = 5 mmol/L) | 55.62 | 74.72 | 36.68 | 6.59 | -20.69 | 111.40 |
| Co3O4@SiO2 (CCTAB =25 mmol/L) | 66.68 | 72.18 | 31.01 | 6.83 | -15.76 | 103.19 |
| Co3O4@SiO2 (VTEOS = 0.8 mL) | 81.68 | 70.60 | 34.06 | 7.12 | -15.97 | 104.65 |
| Co3O4@SiO2 (VTEOS = 2.0 mL) | 73.55 | 64.75 | 27.90 | 6.28 | -18.74 | 92.64 |
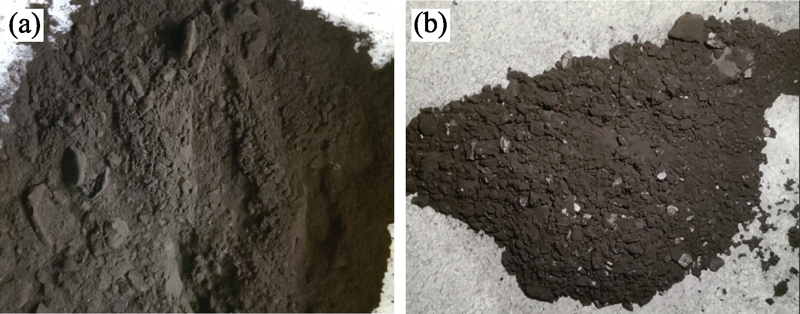
图7 Co3O4@SiO2 (VTEOS = 2.0 mL)反应前后的外观对比图
Fig. 7 Appearance comparison of Co3O4@SiO2(VTEOS = 2.0 mL) before and after the reaction (a) Fresh; (b) 1st cycle
| [1] | 黄瑞丽. 国内环己酮生产现状及消费分析. 合成纤维工业, 2020, 43(4):63-67. |
| [2] | 宋星星, 李永祥, 吴巍. 环己基过氧化氢分解工艺技术现状和发展. 化工进展, 2004, 23(3):322-325. |
| [3] |
IMANAKA N, MASUI T, JYOKO K. Selective liquid phase oxidation of cyclohexane over Pt/CeO2-ZrO2-SnO2/SiO2 catalysts with molecular oxygen. Journal of Advanced Ceramics, 2015, 4(2):111-117.
DOI URL |
| [4] |
KIRILLOVA M V, KOZLOV Y N, SHUL'PINA L S, et al. Remarkably fast oxidation of alkanes by hydrogen peroxide catalyzed by a tetracopper(II) triethanolaminate complex: promoting effects of acid co-catalysts and water, kinetic and mechanistic features. Journal of Catalysis, 2009, 268(1):26-38.
DOI URL |
| [5] |
SAINT-ARROMAN R P, BASSET J M, LEFEBVRE F, et al. Well-defined group IV supported catalysts: an efficient way to increase activities in the deperoxidation of cyclohexyl hydroperoxide with environmentally systems. Applied Catalysis A: General, 2005, 290(1/2):181-190.
DOI URL |
| [6] |
GUO LULU, LI LIXIA, HE PENGCHENG, et al. Mesoporous material Co/SBA-15 as catalyst for the decomposition of cyclohexyl hydroperoxide. Journal of Inorganic Materials, 2017, 32(5):543-549.
DOI URL |
| [7] |
OBAIDULLAHA M, BAHADURB N M, FURUSAWAA T, et al. Microwave assisted rapid synthesis of Fe2O3@SiO2 core-shell nanocomposite for the persistence of magnetic property at high temperature. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2019, 572:138-146.
DOI URL |
| [8] | WU S L, YANG R X, WEY M Y. Catalytic methane decomposition to hydrogen over a surface-protected core-shell Ni@SiO2 catalyst. Chemical Engineering & Technology, 2018, 41(7):1448-1456. |
| [9] |
LIU BING, WANG DEPING, HUANG WENHAI, et al. Preparation of core-shell SiO2/Fe3O4 nanocomposite particles via Sol-Gel approach. Journal of Inorganic Materials, 2008, 23(1):33-38.
DOI URL |
| [10] |
PEJOVA B, ISAHI A, NAJDOSKI M, et al. Fabrication and characterization of nanocrystalline cobalt oxide thin films. Materials Research Bulletin, 2000, 36(2001):161-170.
DOI URL |
| [11] |
ZHAO L, SHI S, LIU M, et al. Hydrophobic modification of microenvironment of highly dispersed Co3O4 nanoparticles for the catalytic selective oxidation of ethylbenzene. ChemCatChem, 2019, 12(3):903-910.
DOI URL |
| [12] |
NEIVANDT D J, GEE M L, HAIR M L. Polarized infrared attenuated total reflection for the in situ determination of the orientation of surfactant adsorbed at the solid/solution interface. J. Phys. Chem. B, 1998, 102(26):5107-5114.
DOI URL |
| [13] |
KIM J H, YOON S B, KIM J Y, et al. Synthesis of monodisperse silica spheres with solid core and mesoporous shell: morphological control of mesopores. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2008, 313-314:77-81.
DOI URL |
| [14] |
YOON S B, KIM J Y, KIM J H, et al. Synthesis of monodisperse spherical silica particles with solid core and mesoporous shell: mesopore channels perpendicular to the surface. Journal of Materials Chemistry, 2007, 17(18):1758-1761.
DOI URL |
| [15] |
YIN L, TIAN Q, BOYJOO Y, et al. Synthesis of colloidal mesoporous silica spheres with large through-holes on the shell. Langmuir, 2020, 36(25):6984-6993.
DOI URL |
| [16] |
DING H L, ZHANG Y X, WANG S, et al. Fe3O4@SiO2 core/shell nanoparticles: the silica coating regulations with a single core for different core sizes and shell thicknesses. Chemistry of Materials, 2012, 24(23):4572-4580.
DOI URL |
| [17] |
TENG Z, SU X, ZHENG Y, et al. Mesoporous silica hollow spheres with ordered radial mesochannels by a spontaneous self-transformation approach. Chemistry of Materials, 2012, 25(1):98-105.
DOI URL |
| [18] |
ZHU H, MA Z, CLARK J C, et al. Low-temperature CO oxidation on Au/fumed SiO2-based catalysts prepared from Au(en)2Cl3 precursor. Applied Catalysis A: General, 2007, 326(1):89-99.
DOI URL |
| [1] | 吴锐, 张敏慧, 金成韵, 林健, 王德平. 光热核壳TiN@硼硅酸盐生物玻璃纳米颗粒的降解和矿化性能[J]. 无机材料学报, 2023, 38(6): 708-716. |
| [2] | 马晓森, 张丽晨, 刘砚超, 汪全华, 郑家军, 李瑞丰. 13X@SiO2合成及其甲苯吸附性能[J]. 无机材料学报, 2023, 38(5): 537-543. |
| [3] | 胡越, 安琳, 韩鑫, 侯成义, 王宏志, 李耀刚, 张青红. RhO2修饰BiVO4薄膜光阳极的制备及其光电催化分解水性能[J]. 无机材料学报, 2022, 37(8): 873-882. |
| [4] | 迟聪聪, 屈盼盼, 任超男, 许馨, 白飞飞, 张丹洁. SiO2@Ag@SiO2@TiO2核壳结构的制备及其光催化降解性能[J]. 无机材料学报, 2022, 37(7): 750-756. |
| [5] | 黎邦鑫, 张骞, 肖杰, 肖文艳, 周莹. Fe增强Ni2(CO3)(OH)2臭氧分解抗湿性与催化性能[J]. 无机材料学报, 2022, 37(1): 45-50. |
| [6] | 郑云,陈亦琳,高碧芬,林碧洲. 磷烯光催化分解水研究进展[J]. 无机材料学报, 2020, 35(6): 647-653. |
| [7] | 李孟夏, 陆越, 王利斌, 胡先罗. Mn3O4@ZnO核壳结构纳米片阵列的可控合成及其在水系锌离子电池中的应用[J]. 无机材料学报, 2020, 35(1): 86-92. |
| [8] | 肖文谦,张静,李克江,邹新宇,蔡昱东,李波,刘雪,廖晓玲. 荔枝状CaCO3@HA/Fe3O4磁性介孔多级微球的制备[J]. 无机材料学报, 2019, 34(9): 925-932. |
| [9] | 苟生莲, 乃学瑛, 肖剑飞, 叶俊伟, 董亚萍, 李武. 碱式氯化镁晶须制备纳米氧化镁热分解动力学研究[J]. 无机材料学报, 2019, 34(7): 781-785. |
| [10] | 宋晶晶, 陈波, 林开利. 核壳结构羟基磷灰石/介孔二氧化硅纳米颗粒的制备及其药物释放研究[J]. 无机材料学报, 2018, 33(6): 623-628. |
| [11] | 周蓓莹, 陈东, 刘佳乐, 江莞, 罗维, 王连军. CuInS2/ZnS核壳结构量子点的水相制备与性能研究[J]. 无机材料学报, 2018, 33(3): 279-283. |
| [12] | 王松灿, 汤枫秋, 王连洲. 光电催化分解水用可见光响应型氧化物光阳极的改性研究进展[J]. 无机材料学报, 2018, 33(2): 173-197. |
| [13] | 李 敏, 洛 园, 许伟佳, 刘家祥. DMFC阳极催化剂Fe3O4@Pt的制备及其催化性[J]. 无机材料学报, 2017, 32(9): 916-922. |
| [14] | 杨 桃, 李 肖, 田晓冬, 宋 燕, 刘占军, 郭全贵. 锂离子电池负极材料Si@C/SiOx的制备及其电化学性能[J]. 无机材料学报, 2017, 32(7): 699-704. |
| [15] | 郭露露, 李立霞, 何鹏程, 袁 霞. 介孔材料Co/SBA-15催化环己基过氧化氢分解的研究[J]. 无机材料学报, 2017, 32(5): 543-549. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||